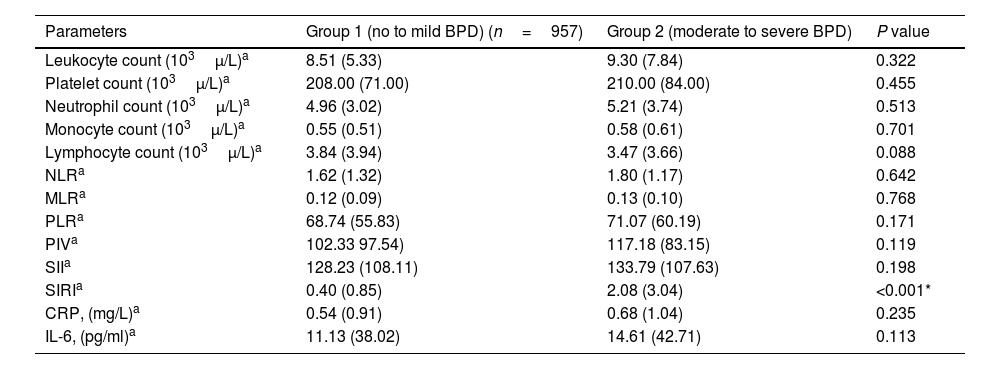
The role of systemic inflammatory indices in the diagnosis of bronchopulmonary dysplasia (BPD) is unknown. The aim of the study was to determine the possible clinical utility of systemic inflammatory indices in the prediction of moderate to severe BPD.
MethodsPremature infants<32 weeks of gestational age were included in the study. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), systemic immune-inflammation index (SII), pan-immune-inflammation value (PIV), and systemic inflammation response index (SIRI) were calculated at birth and at the time of diagnosis of BPD (at 36th weeks of postmenstrual age). The patients were divided into two groups as no or mild BPD and moderate or severe BPD.
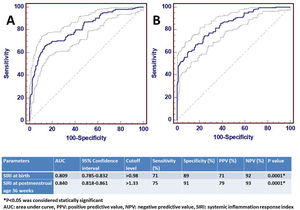
ResultsA total of 1146 infants were included in the study, 957 in Group 1 and 189 in Group 2. The SIRI value was significantly higher in moderate or severe BPD both at birth and at the 36th week of postmenstrual age (p<0.001 and p<0.001, respectively). The AUC value of SIRI was 0.809 and the cut-off value was>0.98 in the predictivity of BPD at birth. The AUC value of SIRI was 0.842 and the cut-off value was>1.33 for the diagnosis of BPD at 36th week of postmenstrual age. After multiple logistic regression analysis, SIRI was shown to be a significant parameter for the diagnosis of BPD (OR 2.847, 95% CI 1.557–4.875).
ConclusionsSIRI may be a useful biomarker for predicting moderate to severe BPD and a marker of clinical importance in the follow-up of infants with BPD.
Bronchopulmonary dysplasia (BPD) is the most common chronic lung disease of infancy, developing secondary to the complex interaction of genetic and environmental factors in premature infants. Invasive mechanical ventilation and usage of supplemental oxygen are two important critical factors among the environmental factors contributing to the pathogenesis of BPD. Despite the heavy burden of the disease, the use of markers to predict BPD in premature infants, is limited.1
The pathogenesis of BPD includes an inflammatory cascade that leads to impaired alveolarization and dysregulated angiogenesis during lung development. Biomarkers that are part of this cascade take important role in the pathogenesis of BPD. Therefore, the use of these biomarkers to effectively predict the risk of BPD continues to be investigated.1 Due to the prominence of inflammation in the pathogenesis of BPD, hematological cells and cytokines are thought to be diagnostic markers. Additionally, it has been stated that some cytokine levels may be valuable parameters in the diagnosis of BPD.2–4 Neonatal hematological parameters have also been shown to be effective markers in the diagnosis of moderate-severe BPD.5–7 However, a specific biomarker that can be used in the diagnosis of BPD has not been determined yet.1
The use of some markers has been shown to be effective for the diagnostic differentiation of respiratory distress in adults. In addition, it has been shown that the systemic inflammatory indices calculated from the complete blood cell count are effectively useful and low-cost parameters in the diagnosis of adult diseases and in determining the clinical outcomes.8–13
Although the predictive role of systemic inflammatory indices in the newborn has been studied in some neonatal diseases except BPD, their place in terms of diagnostic efficiency in these diseases is still unclear.14–19 There is not enough data on whether systemic inflammatory indices are useful parameters in the predictivity of BPD, which causes a heavy disease burden in premature infants. According to the hypothesis of our study, systemic inflammatory indices may be important predictive parameters in diseases such as BPD in which inflammation has an important place in the pathogenesis. For this purpose, neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), pan-immune-inflammation value (PIV), systemic immune-inflammation index (SII), and systemic inflammation response index (SIRI) were investigated in the predictive of BPD in premature infants born below 32 weeks of gestation.
Material and methodsThe study was carried out on newborn babies hospitalized in the neonatal intensive care unit between January 2018 and December 2021. Premature babies with gestational age of <32 weeks were included in the study. Babies with major congenital anomaly, born at >32 weeks of gestation, and born at <32 weeks of gestation and died before the postmenstrual age (PMA) 36th weeks, were excluded from the study. Patient data were obtained retrospectively from the hospital medical records. Ethical approval was obtained from the local ethics committee.
Demographical features and clinical outcomesGestational week (GW), birth weight (BW), administration of antenatal steroid, gender, cesarean section, chorioamnionitis, Apgar scores (at 1st and 5th minutes), duration of respiratory support (mechanical ventilation, non invasive ventilation, oxygen therapy), early onset sepsis (EOS), late onset sepsis (LOS), respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), hemodynamically significant patent ductus arteriosus (hsPDA), necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP), and duration of hospitalization were recorded for all eligible patients.
Definition of BPD and other preterm morbiditiesThose who stopped oxygen support in the first 28 days after birth were defined as non-BPD. Infants who needed>21% oxygen during the first 28 days but did not receive oxygen at 36 weeks of PMA or at discharge were defined as mild BPD. Moderate BPD was defined if a premature infant still needed respiratory support with<30% oxygen at 36 weeks of PMA. If there is a need for positive pressure respiratory support or ≥30% oxygen, it was defined as severe BPD.20 RDS was defined, if there was a need for surfactant in premature infants with respiratory failure.21 Infants with severe IVH (stage≥3) identified by transfontanel ultrasonography were recorded.22 Infants diagnosed with hsPDA by clinical or Doppler echocardiography were recorded.23 Sepsis starting in the first 3 days after birth was defined as EOS and sepsis starting after 3 days was defined as LOS.24 Patients with moderately or advanced (stage≥2) NEC were recorded.25 Infants diagnosed and treated for ROP were recorded.26
Complete blood count and laboratory analysisPeripheral venous blood samples were obtained from all premature infants within the first hour after birth and at the 36th gestational week of PMA (the week at which the diagnosis of BPD was made). Blood samples were taken into an Ethylenediaminetetraacetic acid (EDTA) tube, and after macroscopic coagulation control, complete blood count analysis was performed. Cell-Dyn 3700 automatic hemocytometer (Abbott, Abbott Park, IL, USA) device was used for complete blood count. Complete blood count result, leukocyte count (103μ/L), platelet count (103μ/L) (P), neutrophil count (103μ/L) (N), monocyte count (103μ/L) (M), lymphocyte count (103μ/L) (L) values were recorded. Furthermore, peripheral venous blood samples were centrifuged at 3000rpm for 10min at room temperature. C-reactive protein (CRP) and interleukin-6 (IL-6) levels were analyzed from the obtained serum samples. CRP levels were measured with a Roche Modular P analyzer (CRP latex HS, Roche kit, Roche Diagnostics, GmbH, Mannheim, German). IL-6 level was analyzed by IL-6 solidphase, enzyme-labeled, chemiluminescent sequential immunometric assay on the IMMULITE 1000 analyzer (Siemens Diagnostic Product Corporation, Los Angeles, CA).
Systemic inflammatory indicesThe following methods were used for formulations of systemic inflammatory indexes. NLR=N/L, PLR=P/L, MLR=M/L, SII=P×N/L, SIRI=N×M/L, and PIV=P×N×M/L.14 Babies with non-BPD and mild BPD were included in Group 1, and babies with moderate/severe BPD were allocated as Group 2. Infants in Groups 1 and 2 were compared for demographics, clinical outcomes, complete blood count, and systemic inflammatory indices.
Statistical analysisStatistical Package for Social Sciences (SPSS), version 20.0 (SPSS Inc, Chicago, IL, USA) package program was used for the analysis. Histogram and Kolmogorov–Smirnov test were used to analyze the distribution of the data. Normally distributed data were expressed as mean±standard deviation. Data without normal distribution were given as median and interquartile range (IQR). Categorical variables were given as frequency. Fisher's exact test or Pearson Chi-Square test was used for statistical analysis of categorical data, and t test or Mann–Whitney U test was used for continuous data analysis. A p value of <0.05 was considered statistically significant. Multivariate and ordinal logistic regression was applied to identify the independent risk factors of BPD. The odds ratios (ORs) and 95% confidence interval (CI) were defined in logistic regression analysis. Subsequently, receiver operating characteristics (ROC) curves analysis was performed to evaluate the significance of the statistically significant systemic inflammatory indices. ROC analysis revealed area under the curve (AUC), 95% CI, cut-off levels, sensitivity, specificity, positive predictive value, and negative predictive value levels.
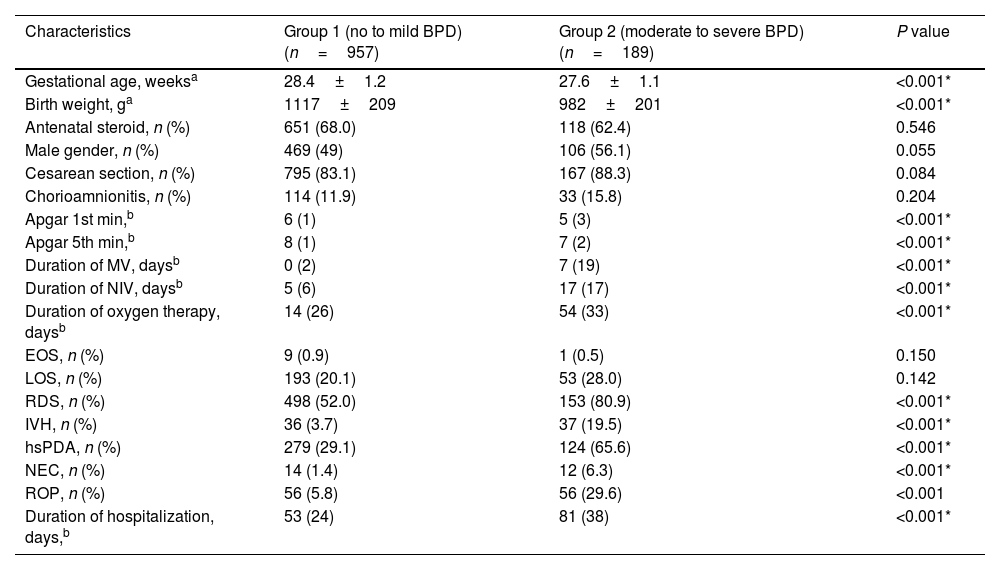
ResultsDuring the study period, a total of 1258 premature infants born at <32 weeks of gestation were hospitalized and followed up in the NICU. Eleven premature infants with major congenital anomalies were excluded from the study. In addition, 101 premature infants who were born at <32 weeks of gestation and died at 36th PMA without a diagnosis of BPD were also excluded from the study. Subsequently, 1146 infants born at <32 weeks of gestation were included in the study. 957 premature infants were diagnosed with non or mild BPD and were included in Group 1. 189 of the remaining premature infants were diagnosed with moderate/severe BPD and were included in Group 2. Antenatal steroid administration, gender, cesarean section, chorioamnionitis, EOS, and LOS frequency were found to be similar between Group 1 and Group 2 (p>0.05). GW, BW, Apgar scores at 1st and 5th minutes were significantly lower in the BPD group (p<0.001 for all characteristics). The duration of respiratory support, RDS, IVH, hsPDA, NEC, ROP, and duration of hospitalization were found to be significantly higher in the moderate/severe BPD group (p<0.001 for all parameters) (Table 1).
Demographic characteristics and clinical outcomes in bronchopulmonary dysplasia.
| Characteristics | Group 1 (no to mild BPD) (n=957) | Group 2 (moderate to severe BPD) (n=189) | P value |
|---|---|---|---|
| Gestational age, weeksa | 28.4±1.2 | 27.6±1.1 | <0.001* |
| Birth weight, ga | 1117±209 | 982±201 | <0.001* |
| Antenatal steroid, n (%) | 651 (68.0) | 118 (62.4) | 0.546 |
| Male gender, n (%) | 469 (49) | 106 (56.1) | 0.055 |
| Cesarean section, n (%) | 795 (83.1) | 167 (88.3) | 0.084 |
| Chorioamnionitis, n (%) | 114 (11.9) | 33 (15.8) | 0.204 |
| Apgar 1st min,b | 6 (1) | 5 (3) | <0.001* |
| Apgar 5th min,b | 8 (1) | 7 (2) | <0.001* |
| Duration of MV, daysb | 0 (2) | 7 (19) | <0.001* |
| Duration of NIV, daysb | 5 (6) | 17 (17) | <0.001* |
| Duration of oxygen therapy, daysb | 14 (26) | 54 (33) | <0.001* |
| EOS, n (%) | 9 (0.9) | 1 (0.5) | 0.150 |
| LOS, n (%) | 193 (20.1) | 53 (28.0) | 0.142 |
| RDS, n (%) | 498 (52.0) | 153 (80.9) | <0.001* |
| IVH, n (%) | 36 (3.7) | 37 (19.5) | <0.001* |
| hsPDA, n (%) | 279 (29.1) | 124 (65.6) | <0.001* |
| NEC, n (%) | 14 (1.4) | 12 (6.3) | <0.001* |
| ROP, n (%) | 56 (5.8) | 56 (29.6) | <0.001 |
| Duration of hospitalization, days,b | 53 (24) | 81 (38) | <0.001* |
BPD, bronchopulmonary dysplasia; EOS, early onset sepsis; hsPDA, hemodynamically significant patent ductus arteriosus; IVH, intraventricular hemorrhage; LOS, late onset sepsis; MV, mechanical ventilation; NEC, necrotizing enterocolitis; NIV, non invasive ventilation; RDS, respiratory distress syndrome; ROP, retinopathy of prematurity.
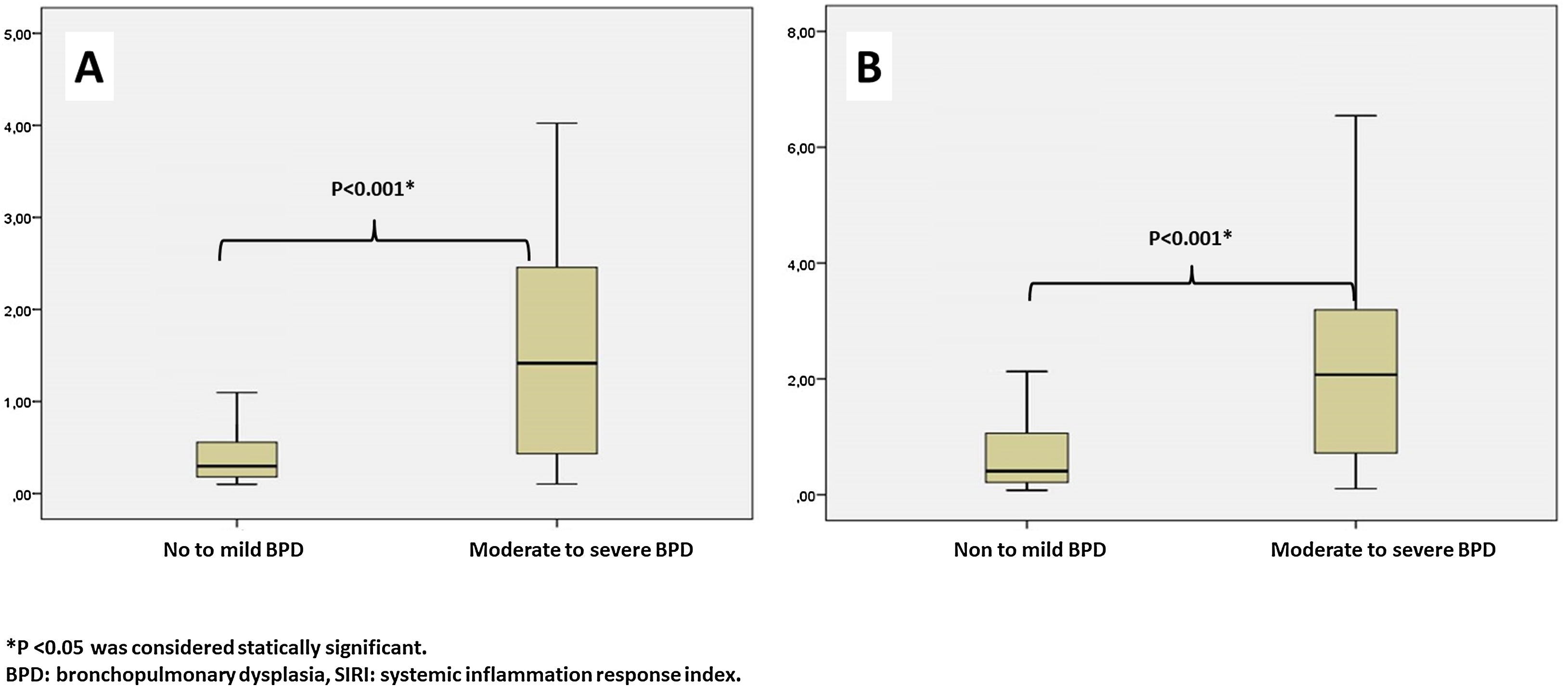
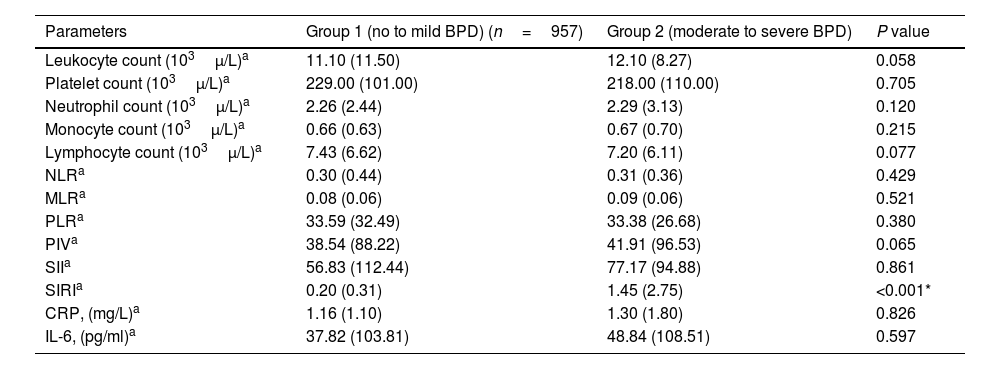
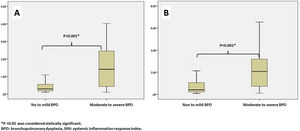
Leukocyte count, neutrophil count, monocyte count, lymphocyte count, platelet count, CRP, IL-6, NLR, MLR, PLR, PIV, and SII values evaluated at both birth and PMA 36th weeks were similar between groups (p>0.05 for all parameters) (Table 2 and Table 3). The SIRI value in Group 2 (median: 1.45) in the blood obtained after birth was found to be significantly higher than the SIRI value (median: 0.2) in Group 1 (p<0.001). The SIRI value in Group 2 (median: 2.08) obtained from blood taken at 36th PMA was significantly higher than the SIRI value (median: 0.4) in Group 1 (p<0.001) (Tables 2 and 3, and Fig. 1).
Systemic inflammatory indices in bronchopulmonary dysplasia at birth.
| Parameters | Group 1 (no to mild BPD) (n=957) | Group 2 (moderate to severe BPD) | P value |
|---|---|---|---|
| Leukocyte count (103μ/L)a | 11.10 (11.50) | 12.10 (8.27) | 0.058 |
| Platelet count (103μ/L)a | 229.00 (101.00) | 218.00 (110.00) | 0.705 |
| Neutrophil count (103μ/L)a | 2.26 (2.44) | 2.29 (3.13) | 0.120 |
| Monocyte count (103μ/L)a | 0.66 (0.63) | 0.67 (0.70) | 0.215 |
| Lymphocyte count (103μ/L)a | 7.43 (6.62) | 7.20 (6.11) | 0.077 |
| NLRa | 0.30 (0.44) | 0.31 (0.36) | 0.429 |
| MLRa | 0.08 (0.06) | 0.09 (0.06) | 0.521 |
| PLRa | 33.59 (32.49) | 33.38 (26.68) | 0.380 |
| PIVa | 38.54 (88.22) | 41.91 (96.53) | 0.065 |
| SIIa | 56.83 (112.44) | 77.17 (94.88) | 0.861 |
| SIRIa | 0.20 (0.31) | 1.45 (2.75) | <0.001* |
| CRP, (mg/L)a | 1.16 (1.10) | 1.30 (1.80) | 0.826 |
| IL-6, (pg/ml)a | 37.82 (103.81) | 48.84 (108.51) | 0.597 |
BPD, bronchopulmonary dysplasia; CRP, C-reactive protein; IL-6, interleukin 6; MLR, monocyte to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; PIV, pan immune inflammation value; PLR, platelet to lymphocyte ratio; SII, systemic immune inflammation index; SIRI, systemic inflammation response index.
Systemic inflammatory indices in bronchopulmonary dysplasia at postmenstrual age 36th weeks.
| Parameters | Group 1 (no to mild BPD) (n=957) | Group 2 (moderate to severe BPD) | P value |
|---|---|---|---|
| Leukocyte count (103μ/L)a | 8.51 (5.33) | 9.30 (7.84) | 0.322 |
| Platelet count (103μ/L)a | 208.00 (71.00) | 210.00 (84.00) | 0.455 |
| Neutrophil count (103μ/L)a | 4.96 (3.02) | 5.21 (3.74) | 0.513 |
| Monocyte count (103μ/L)a | 0.55 (0.51) | 0.58 (0.61) | 0.701 |
| Lymphocyte count (103μ/L)a | 3.84 (3.94) | 3.47 (3.66) | 0.088 |
| NLRa | 1.62 (1.32) | 1.80 (1.17) | 0.642 |
| MLRa | 0.12 (0.09) | 0.13 (0.10) | 0.768 |
| PLRa | 68.74 (55.83) | 71.07 (60.19) | 0.171 |
| PIVa | 102.33 97.54) | 117.18 (83.15) | 0.119 |
| SIIa | 128.23 (108.11) | 133.79 (107.63) | 0.198 |
| SIRIa | 0.40 (0.85) | 2.08 (3.04) | <0.001* |
| CRP, (mg/L)a | 0.54 (0.91) | 0.68 (1.04) | 0.235 |
| IL-6, (pg/ml)a | 11.13 (38.02) | 14.61 (42.71) | 0.113 |
BPD, bronchopulmonary dysplasia; CRP, C-reactive protein; IL-6, interleukin 6; MLR, monocyte to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; PIV, pan immune inflammation value; PLR, platelet to lymphocyte ratio; SII, systemic immune inflammation index; SIRI, systemic inflammation response index.
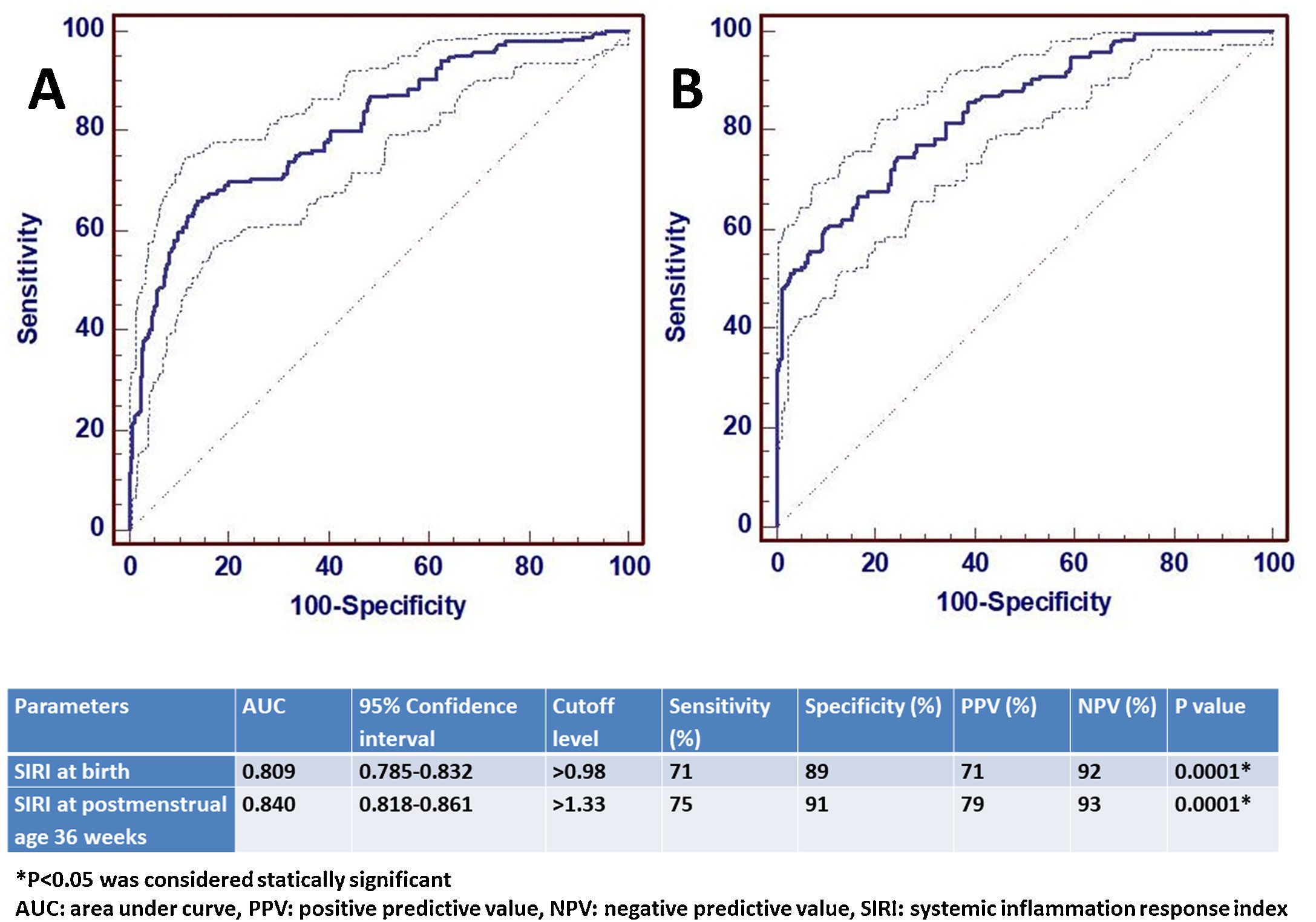
The potential confounder risk factors (GW and BW) were subsequently entered to the multivariable regression model at birth. We found that the risk of moderate/severe BPD was independently associated with BW (OR: 1.304, 95% CI: 1.021–1.647, P=0.001), and GW (OR: 1.844, 95% CI: 1.398–4.101, P=0.002). Multiple analysis showed that a higher level of SIRI (>0.98) was independently associated with BPD (OR 2.847, 95% CI 1.557–4.875).
ROC analysis was performed to determine the statistical significance level of SIRI, which was significant for the diagnosis of moderate/severe BPD at birth and at 36th PMA. The AUC value of SIRI for moderate/severe BPD predictivity at birth was 0.809 and the diagnostic cut-off value was>0.98. For the diagnosis of moderate/severe BPD at the 36th PMA, the AUC value of SIRI was 0.842 and the cut-off value was>1.33. ROC analysis results are shown in Fig. 2.
DiscussionThe current study evaluated systemic inflammatory indices in the predictivity of BPD. To the best of our knowledge, our study is the first to investigate the role and value of six systemic inflammatory indices in the prediction of moderate/severe BPD. We found that NLR, MLR, PLR, PIV, and SII values measured at birth and 36th weeks of PMA, were not systemic inflammatory indices that could be used in the prediction and diagnosis of BPD, respectively. It was found that SIRI value>0.98 at birth and>1.33 at 36th week of PMA was an effective marker in the diagnosis of probable moderate and severe BPD.
Bronchopulmonary dysplasia occurs as a complex process that can lead to serious, lifelong negative consequences by affecting the development of the lower respiratory tract due to various pre/postnatal factors. Because of the inflammatory processes during the formation of BPD, the detection of inflammatory mediators may aid in the early prediction of BPD. However, many biomarkers alone are not disease-specific and may not always be effective in predicting the disease.4 Biomarkers evaluated especially for the predictivity of BPD have generally been studied in cord and peripheral blood, tracheal aspirates and urine. These markers are often evaluated in the first few weeks of life. However, the levels of different biomarkers obtained from blood and branchial spaces may not fully reflect the development of lung parenchymal tissue.1,2,5,6,27,28 Additionally, studies which evaluated the prediction and diagnosis power of the same parameters at birth and at 36th week of PMA, are quite limited.29 In this respect, our study is the first to show that SIRI has a high predictive value in the prediction of BPD both at birth and the diagnosis of BPD at 36th week of PMA. Moreover, the evaluation of systemic inflammatory indices at both birth and 36th week of PMA in the prediction and diagnosis of BPD and the high number of cases are the strengths of our study. We could say that SIRI can be used as a predictor for BPD immediately after birth or as a diagnostic biomarker at 36th weeks of PMA. Therefore, SIRI can be support the diagnosis of BPD when used together with other criteria of BPD.
Neutrophil, monocytes, and lymphocytes, which are the main immunological cells involved in the pathogenesis of BPD.30 Along with proinflammatory cytokineemia, there is a migration of neutrophils and monocytes to the lung tissue. In the early phase, inflammatory cells (neutrophils and macrophages) may contribute to alveolar destruction by secreting proteolytic enzymes.31 On the other hand, T helper cells (Th1 and Th2) help regulate inflammatory responses and maintain inflammatory homeostasis in the lungs.1 There is a finely tuned system of inflammatory control with both pro- and anti-inflammatory mechanisms mediated by T lymphocyte subsets. It has been reported that the number of T lymphocytes decreases in infants with BPD, and this decrease is particularly observed in CD4 and B lymphocytes.32 Thus, the development of BPD occurs with chronic inflammation and injury in the lung tissue.2,27
There are two mechanisms that contribute to the stimulation of immune cells in the pathogenesis of BPD. The first is hypoxia due to lung immaturity, the second is due to respiratory support and oxygen-induced hyperoxia in infants with BPD. Through these two mechanisms, the differentiation of hematopoietic stem cells in the bone marrow is affected, resulting in a change in the number of immune cells.5 Furthermore, the level of free oxygen radicals increases in infants with BPD due to higher oxgene concentrations. Thus, in addition to BPD, premature morbidity increases due to oxidant stress. As a result, a proinflammatory response occurs due to high oxygen and oxidant stress. Increased inflammation also triggers an increase in neutrophil and monocytes series in the bone marrow, and may cause apoptosis in lymphocyte cell lines. Therefore, depending on the severity of hypoxia, hyperoxia and inflammation in BPD patients, there may be numerical and functional changes in neutrophil, monocytes, and lymphocytes cells.33 Although it is known that immune cells have an important role in the pathogenesis of BPD, it is not possible to examine lung tissue samples from preterm infants. Therefore, the roles of immune cells in the pathogenesis of BPD have not been fully elucidated.32
Changes in the number of neutrophil, monocytes, and lymphocyte cells or their functions in peripheral blood can provide information about BPD. In some studies, it has been shown that there may be an increase or decrease in the number of neutrophils, monocytes, and lymphocytes in premature infants with BPD.5,6,28 However, contrary to these results, the change in the number of immune cells may be unrelated to BPD, as found in our study.32 Due to the conflicting results between BPD and the numerical change of immune cells in the literature, it is necessary to examine other inflammatory parameters for the prediction and diagnosis of BPD. Although we did not determine any relationship between the number of neutrophils, monocytes and lymphocytes and the severity of BPD, it was found for the first time that the high SIRI value obtained from the neutrophil, monocytes, and lymphocytes counts was associated with the severity of BPD.
It is known that NLR, PLR, SII, and SIRI values are linearly related to the degree of chronic obstructive pulmonary disease (COPD) exacerbation and lung involvement in the studies evaluating the relationship between COPD and systemic inflammatory indices in adults.34–37 There are limited data on the use of systemic inflammatory indices in the predictive power and diagnosis of the disease in newborns. However, a few studies have been reported that increased NLR, MLR, and PLR values can be used in the diagnosis of neonatal sepsis, ROP, IVH, and NEC.15–18 It has been reported that a high NLR value can be used in the diagnosis of transient tachypnea of the newborn (TTN) in term infants, while PLR has no diagnostic value in TTN.19 The only study related to SIRI in neonates was reported by Ceran et al. performed in term infants with hypoxic ischemic encephalopathy (HIE). According to this study, it was found that SIRI values>1.29 could be used for the diagnosis of HIE.14 In our study, we found that SIRI value>0.98 at birth and>1.33 at 36th week of PMA were associated with the severity of BPD. Sun et al. evaluated the effectiveness of NLR in the predictivity of BPD in 144 premature infants (<32 weeks of gestation) with BPD and 152 infants without BPD, by comparing both groups at birth, at the postnatal 72nd hour, at the 1st week and at the 2nd week. An increase in NLR at birth and at 72nd hours has been reported to be associated with BPD. However, it has been found that NLR was not significant in BPD predictivity at the 1st week, and 2nd week of life.38 As far as we know, the relationship between MLR, PLR, SII, PIV, and, SIRI and the prediction or the diagnosis of BPD has not been evaluated until now. In our study NLR, MLR, PLR, SII and PIV values at birth and 36th weeks of PMA were not found as predictive parameters in the diagnosis of BPD. However, we found that SIRI could be a new inflammatory index for the predictivity at birth and diagnosis of BPD at 36th PMA. Thus, we suggested that SIRI value could be used to determine the severity of BPD.
Hrubaru et al. investigated the predictive value of NLR, MLR, PLR, SII, and SIRI for premature delivery in pregnant women. NLR and PLR values in preterm pregnants were found to be significantly higher than in term pregnants. Additionally, SII and SIRI reported that they did not have a predictive degree for preterm delivery. These results were attributed to the possible association of preterm delivery with maternal neutrophil, lymphocyte and platelet values. Therefore, it has been stated that NLR and PLR may increase in pregnant women due to preterm delivery.39 Our results can be hypothesized that while neutrophil, monocytes, and lymphocyte parameters are not associated with BPD alone, they may become significant when formulated in SIRI together.
Based on our results, it is reasonable to assume that SIRI reflects the severity of BPD more strongly than the number of neutrophils, monocytes, and lymphocytes alone. Thus, the strong predictiveness of SIRI in the diagnosis of BPD would inform the clinician long ago about the most important respiratory problem of the preterm babies. Attending clinicians could predict the respiratory morbidity of preterm infants, and more reliable prognostic information could be given while informing the parents as well. An important advantage of SIRI in the prediction and the diagnosis of BPD is that it can be a fast, low cost and powerful marker. In addition, it can be helpful in differentiating respiratory symptoms that may occur due to non-BPD causes. In this respect, studies evaluating the effectiveness of SIRI should be conducted to distinguish other causes of respiratory problems in preterm infants from BPD. Furthermore, those studies can determine whether SIRI can be a new marker in the management of patients with BPD and in monitoring the response to treatment.
We accept that our study has some limitations as well as its strengths. Due to its retrospective design, other biomarkers that may be associated with BPD could not be evaluated. There have been some new BPD definitions proposed afterward to consider newer modes of non-invasive ventilation that were not included in the previous definitions. In our study, Jobe AH and Bancalari E classification was used for the definition of BPD. Since the national BPD classification based on the criteria created by Jobe AH and Bancalari E was used for the classification of the patients in the unit during the study period. As our study was retrospective, the Higgins RD, et al. definition could not be used for BPD classification at the beginning of the study.40 Systemic inflammatory indices values could not be evaluated at certain intervals during the hospitalization of premature infants. Finally, the findings from the single center data were evaluated. Therefore, we cannot generalize our results for now.
In conclusion, it was found for the first time that only SIRI could have predictive value for the severity of BPD and diagnosis of BPD when six systemic inflammatory indices were examined together at the first day of life and at 36th weeks of PMA. High SIRI levels can be a valuable adjunct parameter in addition to clinical factors for risk stratification of BPD and individualization of treatment options. Further studies are warranted to determine the importance of SIRI for monitoring BPD progression and response to different treatment strategies.
Funding sourceNone.
Conflict of interestNone.
None.