«Evidence-based medicine is the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients(…) Good doctors use both individual clinical expertise and the best available external evidence, and neither alone is enough.» David Sackett (1996)1
Pulmonary embolism (PE), the most serious presentation of venous thromboembolism (VTE), is still the third most common cause of cardiovascular mortality.2 Respiratory disease brings together disorders that are pathophysiologically very distinct from each other, with widely varying spectrums of severity. Respiratory disorders can exert an effect on pulmonary circulation, and this situation has led to a growing interest in understanding more fully their relationship with PE. It has been suggested that hypoxia contributes to the development of VTE in some circumstances such as high altitudes, aeroplane flights and even cancer.3 One molecular mechanism that could explain this is the hypoxia-inducible factor (HIF). HIF is stimulated in situations of hypoxaemia (both sustained and intermittent), with a subsequent activation of the inflammatory cascade mediated by the transcription factor nuclear-κB, which regulates the expression of inflammatory cytokines, adhesion molecules and other pro-thrombotic factors, including the plasminogen activator inhibitor-1 and the tissue factor pathway inhibitor.4 Moreover, hypoxia can also increase platelet reactivity.5 From a prognostic viewpoint, PE can produce an overload of the right-side heart cavities and worsen a ventilation/perfusion disorder, with a potentially negative impact on the underlying respiratory disease. Likewise, the presence of a respiratory disorder is associated with a poorer prognosis for PE.2
Chronic obstructive pulmonary disease (COPD) is considered a moderate risk factor for the development of VTE, specially during exacerbations. Although some classic studies reported the presence of PE in up to 20–25% of patients requiring hospitalization due to COPD exacerbation,6 later studies observed a lower rate (3–6%).7 Interestingly, PE is the most frequent presentation of VTE in COPD, whereas in the general population deep vein thrombosis (DVT) is two times more common than PE.8 The diagnosis of PE in cases of COPD often poses a challenge, due to an overlap between the two conditions’ symptoms. The reduced reserve of COPD patients leads to a greater respiratory compromise in the face of an acute PE, explaining their higher short-term mortality, thereby making a case for the prevention and early identification of this complication in patients with COPD.9 Anyhow, strategies for the active detection of PE via imaging tests must be suitably balanced, bearing in mind the frequency of the detection of isolated subsegmental PE and the additional risk resulting from anticoagulation. In fact, one recent multicentre randomized clinical trial found that an active PE detection strategy based on D-dimer values did not show any benefit in patients hospitalized for COPD exacerbation.7
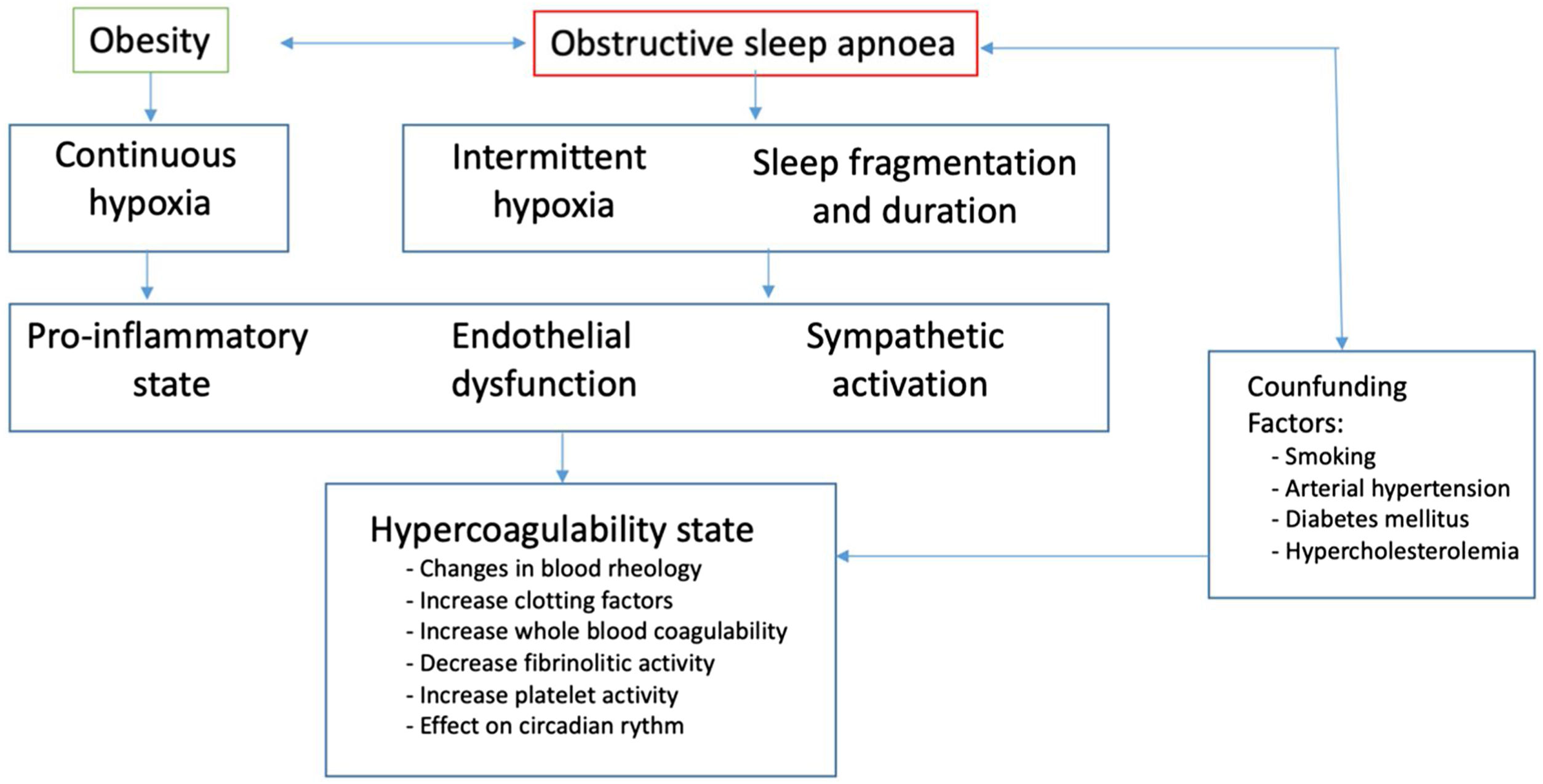
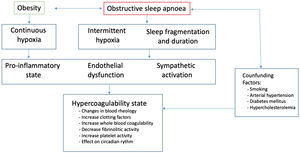
Obstructive sleep apnoea (OSA) is involved in the pathogenesis of cardiovascular diseases, but its association with PE has been studied in less depth. Although a causal relationship between OSA and the development of VTE has not yet been demonstrated, there is growing evidence pointing to OSA as a risk factor for PE. OSA has been associated with the main known etiopathogenic mechanisms of VTE: Virchow's triad (hypercoagulability, endothelial disfunction and venous stasis) (Fig. 1).10 Epidemiological studies have found higher rates of VTE in OSA patients, and case–control studies have identified a two-threefold increased risk of VTE. The prevalence of OSA, and its severity, as evaluated by the apnoea-hypopnoea index (AHI), in patients with PE is higher than would be expected in the general population. In fact, the risk of presenting at least a moderate OSA (i.e. AHI>15/hour) is four times higher in PE patients,11 supporting the importance of registering a suitable sleep history in these circumstances. Current evidence suggests that OSA is associated with a poorer prognosis for PE, as both a higher risk of death and an increase in VTE recurrences has been observed in OSA patients.12,13 More studies are needed to elucidate the complex two-directional relationships between OSA and PE, including the role of CPAP.
The interstitial lung diseases (ILD) constitute a heterogeneous group embracing over 200 conditions. Our knowledge of their relationship with VTE is fairly sparse. Thromboembolic complications are common in patients with ILD.14 Case–control studies have observed a threefold risk of presenting VTE in patients with idiopathic pulmonary fibrosis, while populational studies of sarcoidosis patients have also found an increased risk of presenting VTE.15 PE is more common than DVT, which could be explained by anomalies in pulmonary blood vessels caused by certain chemokines and interleukins characteristic of ILD. Due to overlapping of symptoms, the current recommendation is to consider ruling out PE when a respiratory deterioration unaccompanied by any radiological or functional progression.15 Computed tomography pulmonary angiography is usually preferred over ventilation/perfusion scintigraphy for confirming PE, and it also provides relevant information on the radiological evolution of ILD. Interestingly, some authors put forward the hypothesis of a link between PE and the progression of ILD, due to a procoagulant state and a stimulus to the fibrogenic pathways by certain procoagulant enzymes. However, various anticoagulation strategies in patients without confirmed VTE have proved to have a negative prognostic impact on ILD as regards both progression and survival.16 It should be noted that antifibrotic therapies have been associated with an increased risk of bleeding when used in anticoagulated patients because of its mechanism of action.17
COVID-19 has been associated with a state of inflammation and hypercoagulability, especially in patients requiring hospitalization and in severe forms of the disease accompanied by acute respiratory distress syndrome (ARDS). It has been explained by an immunothrombosis phenomenon, whereby an excessive innate immune response leads to endothelial damage and activation of the coagulation cascade. In addition, acute hypoxaemia predisposes them to develop micro- and macro-thrombotic events – especially in those with ARDS.18 One meta-analysis that included almost 20,000 patients found a high incidence of VTE in patients hospitalised for COVID-19 (17%), even though most patients were receiving pharmacological thromboprophylaxis.19 The limited evidence initially available led to the publication of various documents (with striking differences between them) recommending anticoagulation strategies considering specific individual characteristics, analytical data and the severity of the infection. Accordingly, several trials have been carried out simultaneously, mainly in hospital settings, to investigate the role of anticoagulation as antithrombotic prophylaxis and treatment for COVID-19. Results in one multiplatform clinical trial showed that therapeutic-dose heparin in non-critical hospitalised patients was associated with both better survival and a reduced need for respiratory or cardiac support, in comparison with low-dose heparin; however, full-dose anticoagulation increased the haemorrhagic complications.20 These results have supported that full-dose heparin could be considered for patients hospitalised due to COVID-19 who need low-flow oxygen therapy and present high D-dimer values and low risk of bleeding. Anyhow, fuller and more up-to-date information is still required with respect to the various severity profiles of COVID-19, with special attention to the pandemic's current picture (i.e. extensive vaccine coverage, better targeted antivirals and emergence of new, milder variants of SARS-CoV-2).
There are still sizable gaps in our understanding of the epidemiological and pathophysiological relationship between PE and respiratory disorders (both those mentioned above and others such as neuromuscular diseases). The habitual overlap of symptoms of acute PE with several acute respiratory disorders, as well as with exacerbations of chronic respiratory diseases, justifies the need to optimize the diagnosis of PE, and thus the capacity to positively influence the prognosis. Priority must be given to strategies aimed at preventing VTE in patients with underlying respiratory comorbidities, owing to the negative impact of each of these conditions on the other and the subsequent poorer prognosis. An optimal therapeutic control of the respiratory disease could thus reduce the risk of VTE (e.g., reduction of exacerbations, treatment of night-time breathing disorders, etc.). Answers to the many crucial questions still unresolved by respiratory medicine in this respect could therefore have a potentially valuable clinical impact and open up new avenues of knowledge. Such opportunities would require collaboration between the groups working on PE and their counterparts working on the respiratory diseases involved.