Patients with obesity hypoventilation syndrome (OHS) need treatment with positive pressure either with continuous (CPAP) or double pressure (NIV). The apnea–hypopnea index (AHI) is considered a key data for making therapeutic decisions. We hypothesized that HR may be an useful tool to establish different phenotypes and individualize treatment in patients with OHS. Our objective was to analyze the role of the respiratory center response to hypercapnia (HR) in the adequacy of positive airway pressure therapy.
MethodWe included subjects with OHS treated with CPAP or NIV according to AHI and baseline pCO2. We analyzed therapeutic effectiveness and treatment changes prioritizing CPAP if AHI>30/h. Therapy was considered adequate if it was effective after two years. HR was measured with the p0.1/pEtCO2 ratio and its capability to select therapy was analyzed. The statistical study was performed by means comparison (Student's t) and multivariate analysis (logistic regression).
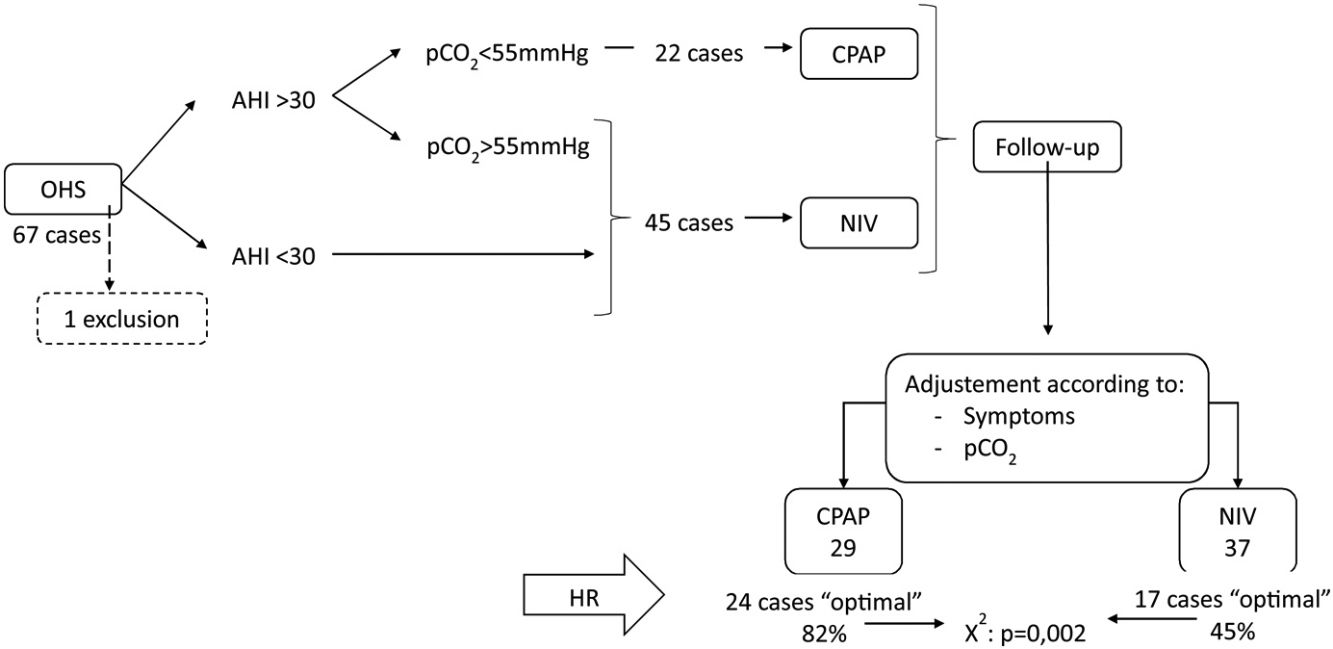
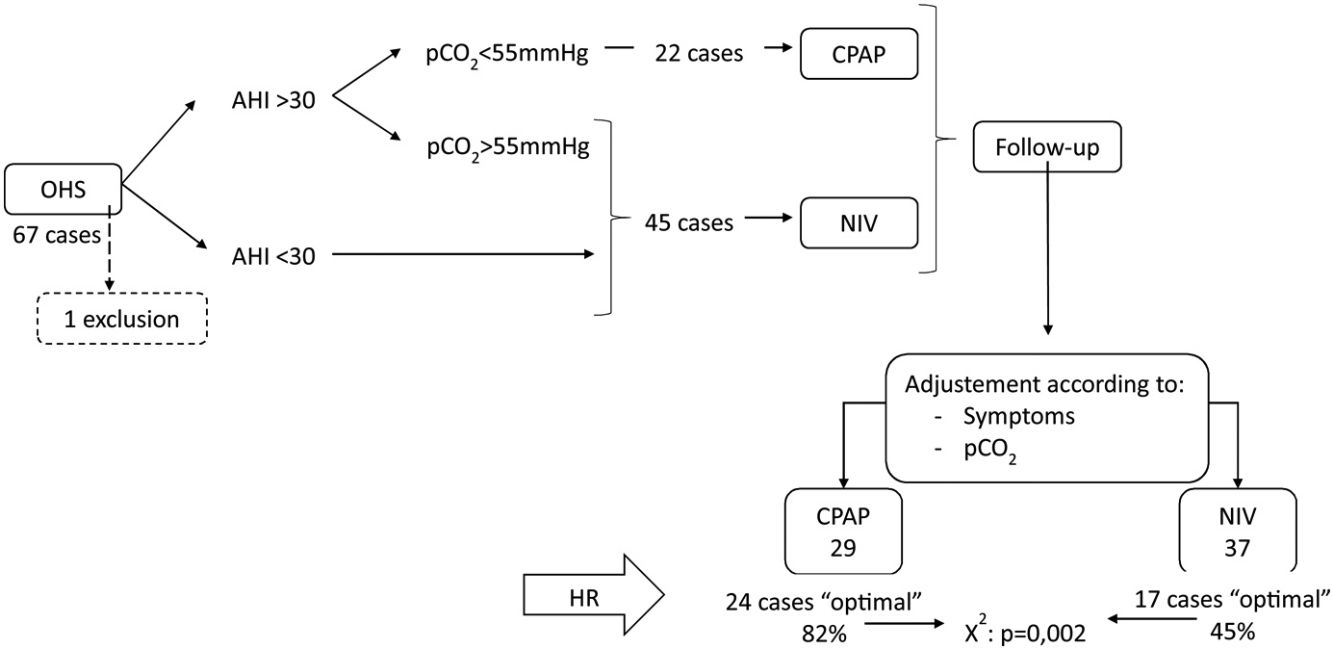
Results67 subjects were included of 68(11) years old, 37 (55%) males, initially 45 (67%) treated with NIV and 22 (33%) with CPAP, one case was excluded and in 25 (38%) the treatment was changed. Finally, CPAP was adequate for 29 subjects (44%) and NIV for 37 (56%). The CPAP group showed AHI 57/h (24) and p0.1/pEtCO2 0.37cmH2O/mmHg (0.23), NIV group AHI 43/h (35) and p0.1/pEtCO2 0.24 (0.15) with p=0.049 and 0.006. In multivariate analysis, p0.1/pEtCO2 (p=0.033) and AHI>30 (p=0.001) were predictors of adequate therapy.
ConclusionMeasuring the RH of the respiratory center helps to select the most appropriate treatment for patients with OHS.
Obesity-hypoventilation syndrome (OHS) is defined as the presence of chronic daytime hypercapnia (PaCO2≥45mmHg) and obesity (BMI≥30kg/m2), after ruling out other causes of hypoventilation.1–5 Its pathogenesis has a multifactorial origin: alteration in ventilatory mechanics, leptin resistance, upper airway obstruction and central hypoventilation.6 The presence of a nocturnal ventilatory disorder is common, which in most cases is a sleep apnea syndrome (OSA),7 although there may be nocturnal hypoventilation without the presence of OSA.8,9
In the treatment of OHS, positive airway pressure is used during sleep, either in the form of continuous pressure (CPAP) or double pressure: non-invasive ventilation (NIV). The choice of one or other device influences both the cost of the treatment and the comfort of the patient, given that in general CPAP is better tolerated and more economical. Currently, it is considered that in patients with severe associated OSA the first therapeutic option is CPAP,10,4 whereas NIV is recommended for those cases where an associated hypoventilation factor is present,11,12 although this factor is not clearly defined.
Moreover, patients with OHS have some characteristics as the level of severity,13 the functioning of their respiratory center,14 the coexistence of heart failure,15 the evolution of the symptoms or the PAP adherence that may influence the clinical course and can lead to changes in the initial therapeutic decision with the intention of adapting the initially therapy prescribed. We hypothesized that the existence of hypoventilation phenomena and specifically respiratory center (RC) dysfunction is a relevant factor in the evolution of these patients and in their therapeutic needs. Our objective was to analyze the role of the respiratory center response to hypercapnia (HR) in the adequacy of positive airway pressure therapy modality in a cohort of patients with OHS.
MethodWith a prospective and observational design, patients diagnosed with OHS who were evaluated in the Non-Invasive Ventilation Ambulatory Unit of our center between 2018 and 2020 were consecutively included. The inclusion criteria were: age≥18 years old and diagnosed with OHS (BMI≥30kg/m2 and daytime hypercapnia with PaCO2>45mmHg).3–5 Patients with severe airflow obstruction, defined as FEV1<50% of predicted value plus FEV1/FVC<70, and other pathologies than can cause hypercapnic respiratory failure, such as neuromuscular diseases or rib cage disorders, were excluded.
The variables analyzed were: sex, age, body mass index (BMI), lung function values: spirometry (FEV1, FVC, FEV1/FVC), lung volumes: residual volume (RV), total lung capacity (TLC), daytime arterial blood gases (pO2, pCO2, pH, HCO3) and data obtained from sleep studies (respiratory polygraphy): apnea–hypopnea index (AHI), percentage of time with SpO2<90% (T90) and mean SpO2.
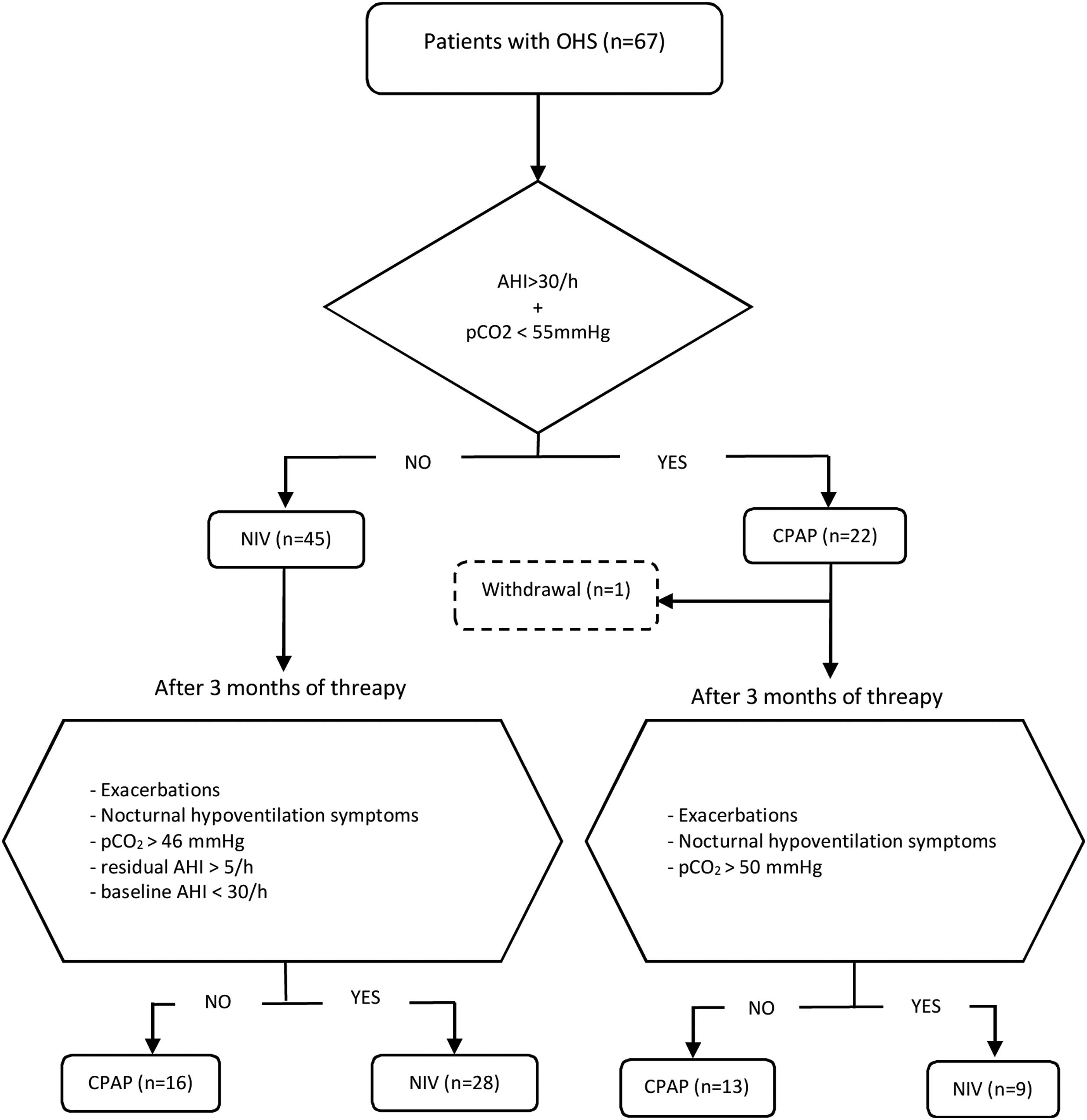
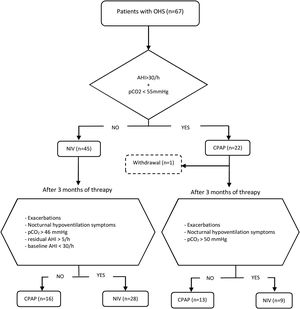
Initial therapy was CPAP in subjects presenting an AHI≥30/h and daytime pCO2<55mmHg, and NIV in those with AHI<30/h and/or daytime pCO2≥55mmHg. This initial treatment was maintained for at least of 3 months. CPAP was initially prescribed at 8 cmH2O and subsequently titrated using autoCPAP devices (S9-Resmed) for at least three nights, adjusting the pressure after manual analysis of pressure curves and taking into account the absence of leaks and residual events. After adjusting the CPAP pressure, pulse oximetry is performed to check T90<30%. The autoCPAP pressure range is 4/20.
The process of adaptation to NIV was carried out with the usual methodology in our unit on an outpatient regimen.14 All PAP therapies were ambulatory adapted in ST mode with S9 VPAP ST or Lumis (ResMed), we used successive visits (15 days, 1 month and 3 months) with trial-and-error approach to set IPAP and EPAP looking for patient comfort, correcting daytime hypercapnia, maintaining an AHI<5/h measured with the device built-in software (BIS) and a mean leak value less than 30lpm. The frequency was programmed in 2 breaths below the spontaneous respiratory rate.
Patients were followed up for a minimum of 2 years, during that period evaluations were carried out with the intention of verifying the effectiveness of the treatment and its adherence. A clinical interview, arterial blood gases and BIS analysis were routinely performed. The treatment was considered adequate if the patient had not presented exacerbations in hypercapnic respiratory failure, used the PAP therapy for more than 4h/day, had improved the symptoms of hypoventilation (such as morning headaches, daytime sleepiness, difficulty concentrating and poor sleep quality), pCO2<46mmHg in daytime blood gases was achieved, and residual AHI was less than 5/h.
Subjects initially treated with NIV with an AHI at diagnosis≥30/h who in their evolution had pCO2<46mmHg, residual AHI<5/h, and had not presented exacerbations, were switched to CPAP, setting it at the EPAP value that have been titrated. On the other hand, individuals initially treated with CPAP who showed symptoms of hypoventilation, exacerbations in hypercapnic respiratory failure and/or persistence of elevated daytime pCO2 values (pCO2>50mmHg) were proposed to change to NIV. Patients who discontinued PAP adherence and those with PAP adherence of less than 4h/day were excluded from the analysis. The follow-up of each individual was carried out for a minimum of two years and the therapy received at the end of that period was considered adequate if all the effectiveness criteria mentioned were accomplished.
The RC study was performed by hypercapnia response (HR) by rebreathing, according to a modification of the method described by Read16 using the Hyp’Air compact+Muscle Study device (Medisoft), measuring the occlusion pressure in the first 100ms of the inspiration (p0.1) while the patient breathes a hyperoxic mixture with 7% CO2, and performing occlusions up to a final pEtCO2 of 70mmHg. The HR results are shown as the slope of the least squares regression line resulting from p0.1/pEtCO2 in cmH2O/mmHg. The ventilatory response to hypercapnia was categorized for analysis as optimal response and suboptimal response using the threshold of 0.22cmH2O/mmHg according to a previous study by our group.14 The RC study was made at baseline and repeated after at least 3 months of PAP therapy (either CPAP or NIV) in case of suboptimal response looking for a possible improvement of the RC function.14
The results are shown as mean and SD in quantitative variables and as percentages in qualitative. The normality distribution of the variables were checked with the Kolmogorov–Smirnov test, analysis was performed using means contrast (Student's t) for quantitative variables and chi-square for qualitative variables, considering p<0.05 statistically significant. To calculate the sample size, the number of patients with a diagnosis of OHS under positive pressure therapy with CPAP or NIV was used as reference; the outcome used was the final therapy (CPAP/NIV) and a probability of 50% was attributed to each event, so that for a confidence level of 95%, with an error of 10%, the required sample is of 23 subjects in every group. A step-by-step logistic regression analysis was performed, being the dependent variable the final treatment considered adequate (CPAP or NIV), and the independent variables those used to grade the severity of the OHS: BMI, AHI>30 and pCO2 at diagnosis13 to which the value of the HR analysis was added and those that showed significance in the bivariate analysis.
The study was approved by the Research Ethics Committee of the Principality of Asturias and informed consent was requested from all participants.
ResultsA total of 67 patients aged 68 (SD: 11) years old, 37 (55%) males, were included in the study. It was decided to start with CPAP therapy in 22 subjects with a mean pressure after titration of 11 (2)cmH2O; and NIV was used in 45 subjects with an inspiratory positive pressure (IPAP) of 18 (2)cmH2O and an expiratory positive pressure (EPAP) of 9 (2)cmH2O, the mean respiratory rate (RR) was 14 (3)bpm.
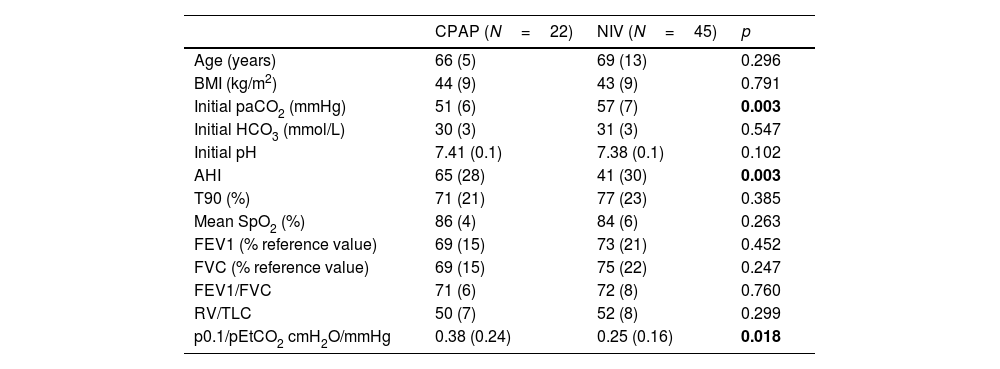
Table 1 shows the functional and anthropometric characteristics of the patients, based on the initial therapeutic decision.
Functional and anthropometric characteristics of the subjects included according to the initial therapeutic decision.
| CPAP (N=22) | NIV (N=45) | p | |
|---|---|---|---|
| Age (years) | 66 (5) | 69 (13) | 0.296 |
| BMI (kg/m2) | 44 (9) | 43 (9) | 0.791 |
| Initial paCO2 (mmHg) | 51 (6) | 57 (7) | 0.003 |
| Initial HCO3 (mmol/L) | 30 (3) | 31 (3) | 0.547 |
| Initial pH | 7.41 (0.1) | 7.38 (0.1) | 0.102 |
| AHI | 65 (28) | 41 (30) | 0.003 |
| T90 (%) | 71 (21) | 77 (23) | 0.385 |
| Mean SpO2 (%) | 86 (4) | 84 (6) | 0.263 |
| FEV1 (% reference value) | 69 (15) | 73 (21) | 0.452 |
| FVC (% reference value) | 69 (15) | 75 (22) | 0.247 |
| FEV1/FVC | 71 (6) | 72 (8) | 0.760 |
| RV/TLC | 50 (7) | 52 (8) | 0.299 |
| p0.1/pEtCO2 cmH2O/mmHg | 0.38 (0.24) | 0.25 (0.16) | 0.018 |
BMI=body mass index; HCO3=calculated from blood gas sample; AHI=apnea–hypopnea index; T90=total record time below 90% oxygen saturation in respiratory polygraphy; FEV1=forced expiratory volume in the first second; FVC=forced vital capacity; RV=residual volume; TLC=total lung capacity; P0.1=occlusion pressure alt 100ms; pEtCO2=end-tidal carbon dioxide pressure; mean SpO2=mean SpO2 in respirator polygraphy. Bold values are the statistically significant results.
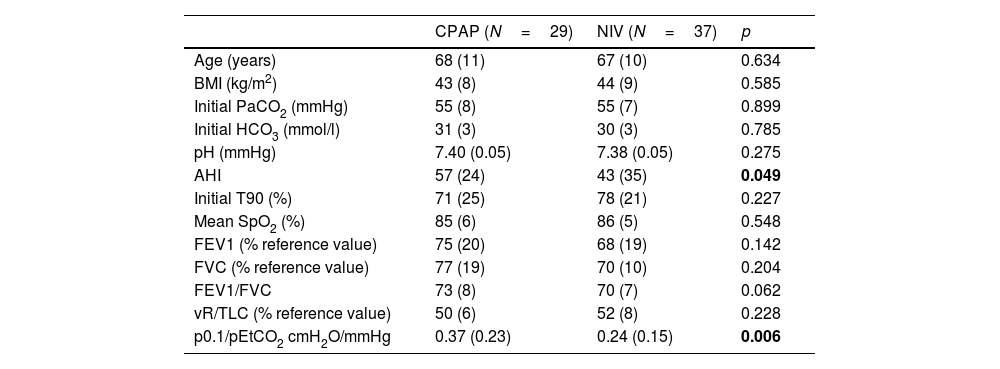
During the follow-up period one patient in the CPAP group was excluded of the final analysis for having an adherence below 4h/night and twenty-five treatments were changed (38%) (Fig. 1). Of the 22 patients with initial CPAP treatment 9 were switched to NIV treatment due to persistent daytime hypercapnia (pCO2>50mmHg), exacerbations, nocturnal hypoventilation symptoms or a combination of these; while 16 of the 45 patients with initial NIV treatment were switched to CPAP due to lack of hypoventilation symptoms, pCO2<45mmHg and residual AHI<5 in BIS. After changes, CPAP was the appropriate therapy for 29 subjects (44%) and NIV for 37 (56%). The mean pressure of the CPAP was 10 (2) cmH2O and in NIV the mean pressures were IPAP 18 (2)cmH2O, EPAP 9 (2)cmH2O, with a mean RR of 14 (2)bpm. Table 2 shows the characteristics of the individuals based on the final treatment.
Functional and anthropometric characteristics of the subjects included according to the final treatment.
| CPAP (N=29) | NIV (N=37) | p | |
|---|---|---|---|
| Age (years) | 68 (11) | 67 (10) | 0.634 |
| BMI (kg/m2) | 43 (8) | 44 (9) | 0.585 |
| Initial PaCO2 (mmHg) | 55 (8) | 55 (7) | 0.899 |
| Initial HCO3 (mmol/l) | 31 (3) | 30 (3) | 0.785 |
| pH (mmHg) | 7.40 (0.05) | 7.38 (0.05) | 0.275 |
| AHI | 57 (24) | 43 (35) | 0.049 |
| Initial T90 (%) | 71 (25) | 78 (21) | 0.227 |
| Mean SpO2 (%) | 85 (6) | 86 (5) | 0.548 |
| FEV1 (% reference value) | 75 (20) | 68 (19) | 0.142 |
| FVC (% reference value) | 77 (19) | 70 (10) | 0.204 |
| FEV1/FVC | 73 (8) | 70 (7) | 0.062 |
| vR/TLC (% reference value) | 50 (6) | 52 (8) | 0.228 |
| p0.1/pEtCO2 cmH2O/mmHg | 0.37 (0.23) | 0.24 (0.15) | 0.006 |
BMI=body mass index; HCO3=calculated from blood gas sample; AHI=apnea–hypopnea index; T90=total record time below 90% oxygen saturation in respiratory polygraphy; FEV1=forced expiratory volume in the first second; FVC=forced vital capacity; RV=residual volume; TLC=total lung capacity; P0.1=occlusion pressure alt 100ms; pEtCO2=end-tidal carbon dioxide pressure; mean SpO2=mean SpO2 in respirator polygraphy. Bold values are the statistically significant results.
In the RC study, 41 cases (62%) showed an optimal response and 25 (38%) a suboptimal response. Of the 29 subjects adequately treated with CPAP 24 (82%) had optimal response, and of the 37 treated with NIV, only 17 (45%) had optimal response (chi-square p=0.002).
In the multivariate logistic regression analysis using a stepwise approach, only baseline AHI>30 (p=0.001) and HR (p=0.033) were independently associated with de primary outcome of adequate response to therapy. BMI and baseline pCO2 level were not associated with response to therapy (Table 3).
DiscussionIn our series of patients with OHS and treatment with positive pressure, the therapeutic modality that seemed most appropriate in long-term treatment was CPAP in 29 (44%) of the cases and NIV in 37 (56%). The factors related to the adequacy of the treatment were the severity of the OSA and the respiratory center function. The HR study revealed a hypoventilation factor in 38% of the subjects that could suggest the need to prioritize NIV.
Although there is evidence indicating that in patients with OHS with severe OSA, CPAP is the first therapeutic option,6,7,12 in our study we have detected a subgroup of these patients who did not respond adequately to CPAP, and some of them showed a RC dysfunction; on the other hand, the group of patients who required NIV at the end of the follow-up showed an AHI mean value of 43 (SD:35) which indicates that in a significant number of cases with severe OSA, CPAP was not adequate and NIV was required. The reference studies show differences in the characteristics of the patients or in the design, thus the pCO2 values are lower at inclusion,6 or the follow-up times are shorter,13 which avoids the analysis of therapeutic changes. In our study changes were made in 25 cases (38%), in 16 subjects CPAP therapy was prescribed18,19 while in 9 subjects treatment with CPAP was considered inadequate, due to persistent daytime hypercapnia or exacerbation leading to hospital admissions, and NIV was prescribed.4
In the initial management of patients, we have chosen NIV in those individuals who presented a higher initial pCO2 value, being suggestive of greater component of hypoventilation15 that would require NIV. Other consensus documents also suggest taking into account advanced age, or lung function data.4,12 However, a recent study20 found no differences in the evolution of pCO2 based on the prescribed therapeutic modality, and our results did not detect a relationship between the initial pCO2 value and the adequate therapy.
The function of the RC has been analyzed in subjects with OSA or COPD18,19 at baseline and after CPAP or NIV, and it has been detected a tendency to present low response to hypercapnia and improvement after pressure therapy. In a previous study carried out by our group in patients with OHS14 we showed different subgroups in relation to RC function suggesting the existence of different phenotypes in OHS with possible implications in the therapy options. One of the strengths of our study is the inclusion of the function of the respiratory center in the assessment of these patients in a “real life” scenario, including a long-term follow-up and an analysis of therapeutic changes based on effectiveness. Our results detect the existence of a group of subjects with RC dysfunction both in the presence of severe OSA and in its absence, and NIV would be more necessary in this subgroup of patients than in those with an adequate RC function, and allows us to hypothesize that this data may be a relevant hypoventilation factor useful in making treatment decisions. Our works presents some limitations, firstly the lack of randomization in the design, the number of patients included is limited, it is a one-center study, and the lack of objective nocturnal pCO2 measure to check the effectiveness of the treatment.
In conclusion, in our series of patients with OHS and treatment with positive pressure, we detected a subgroup with a respiratory center dysfunction which presence shown a predictive capacity to determine the appropriate long-term therapy. Incorporating the functional study of the respiratory center in subjects with OHS could help to establish different phenotypes in this pathology and constitute a useful tool to individualize the treatment to be used.
FundingThere were no funding sources.
Conflict of interestsThe authors state that they have no conflict of interests.