Chronic obstructive pulmonary disease (COPD) is a highly prevalent chronic process1 that presents a real challenge for health care systems. Numerous factors have contributed to the growing problem, such as tobacco use at earlier ages, increased numbers of active smokers, and environmental pollution. The aging population, which presents multiple associated pathologies and consumes enormous health resources, also plays a role in rising physical and financial health costs.2
The rate of underdiagnosis is high,1 as is the degree of disability caused by COPD, which exerts considerable pressure on hospitals and primary care.3,4 The difficulties in treating and following up these patients make it necessary to develop clinical practice guidelines (CPGs) that guarantee adequate continuity of care when patients transition to different levels of care.
Despite great efforts to improve the management of patients with COPD, the low implementation of spirometry4,5 and the irregularity and heterogeneity of follow-up continue to be two particularly critical points to be resolved. In addition, there are other important issues to address, such as adherence to CPGs, promotion of health education and air quality control.2 However, the recognized role of forced spirometry in the diagnosis of the disease, as well as its limited use, are topics that are widely addressed in different documents and CPGs.6–8 Furthermore, the information that we can obtain about how patients should be monitored beyond a therapeutic approach is scarce.
With the intention of improving the continuity of care for these patients, different integrated care processes (ICPs) have been prepared with little success. Focusing specifically on monitoring patients with COPD, the health care schemes proposed by ICPs are quite rigid. For example, the assessment intervals are based on the severity of the process (e.g., degree of obstruction to airflow) and risk of complications as determined by the Spanish Guide of Chronic Obstructive Pulmonary Disease (GesEPOC) and are not adjusted according to the individual patient's clinical changes. Furthermore, recommendations on the follow-up care of patients with stable COPD have little bibliographic support, given the contradictory results of studies that have evaluated different “care formulas”.2,9,10
To facilitate the continuity of care for patients with COPD, the document “Referral criteria for COPD: Continuity of care”11 is a practical guide that has been prepared by scientific societies whose members care for patients with this disease. The guide describes in detail the initial diagnostic assessment, the treatment of the patient with COPD and follow-up. With respect to this last point, the objective should be to guarantee the well-being of the patient and optimize the available resources, so collaboration and communication between hospitals and primary care must be fluid. However, both in COPD and in other respiratory diseases, patients’ transition from hospitals to primary care providers has not been smooth. Instead, patients have not received comprehensive care, and scheduled consultations have been delayed, necessitating frequent visits to the emergency room; furthermore, miscommunications have led to duplicated consultations and tests.2 To resolve these problems, we consider that there are four key points to assess11: control of symptoms, prevention of exacerbations/search for aggravations, continuity in the evaluation of the inhalation technique/adherence to treatment and the monitoring of lung function.
From an organizational point of view, the improvement of symptoms and especially the prevention of exacerbations should determine follow-up intervals. The natural history of COPD is punctuated by frequent periods of exacerbation, which in some cases lead to hospitalization.12 These episodes produce a substantial deterioration in the quality of life of the patient, an increase in their risk of morbidity and mortality, and great health care costs.2 Consequently, identifying the patients who are most susceptible to exacerbation is a priority. The questionnaire proposed by GesEPOC in its 2021 edition6 has proven to be sensitive to clinical changes in the patient and is capable of predicting exacerbations in a relatively short period. Thus, those patients with COPD labeled as “poorly controlled” have a greater risk of suffering an exacerbation in the following 3–6 months,13 a situation that would support the need to reduce follow-up intervals. These changes are not exclusive to patients with more advanced COPD (high risk according to GesEPOC 2021) but may also be present in patients with less complexity (low risk according to GesEPOC 2021).14 Therefore, the degree of control can be a tool in the management of patients regardless of the severity of the disease.
Factors such as the presence of comorbidities (especially those of a cardiovascular nature), the social situation of the patient, failures in the inhalation technique or poor adherence to CPGs have been related to a greater deterioration in the patient's quality of life, missed primary care visits and an increase in health spending.2 All these aspects should be reviewed at each visit, especially for individuals for whom clinical control has not been achieved.
For patients who are fragile or have mobility issues, telephone follow-up could be a complementary activity to face-to-face visits,9 allowing health care providers to promote self-management, regular physical activity or early recognition of an exacerbation.
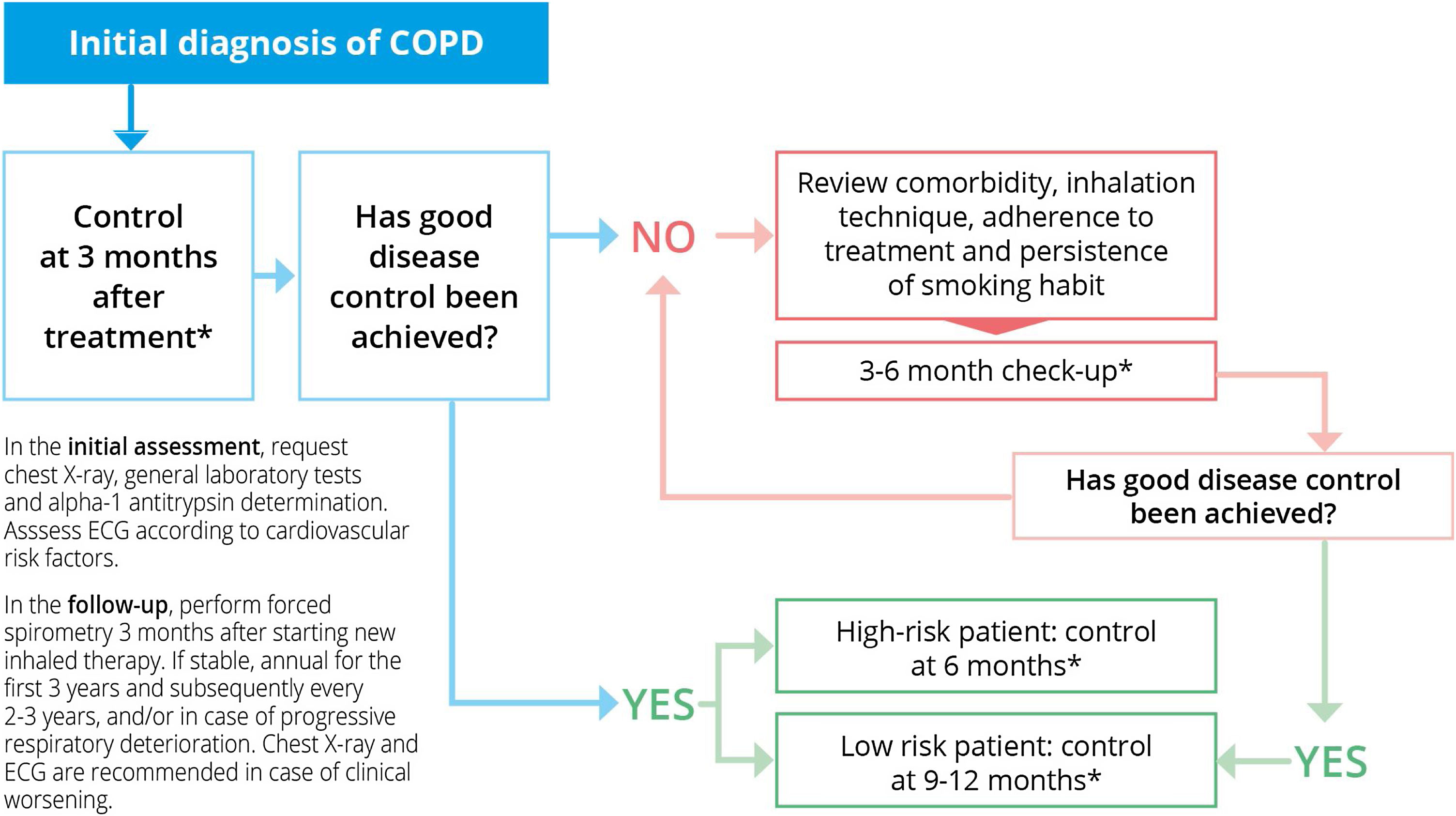
Information on lung function monitoring is scarce.6–8 Current evidence supports the performance of an annual physical examination in the first three years after diagnosis, with the intention of identifying those patients with an accelerated decline in lung function. Beyond this recommendation, the evidence is insufficient. Forced expiratory volume in the first second (FEV1) has been recognized as an important prognostic factor. Therefore, our opinion is that follow-up visits to take this measurement should be carried out every two to three years,15 unless changes are made to the treatment, in which case consultations should be conducted every three months. FEV1 and chest X-ray are recommended in situations of clinical deterioration or only slight improvement after the appropriate treatment or an adjustment to treatment. Based on all this, the document on referral criteria for COPD proposes the scheme shown in Fig. 1.
Follow-up of patients with stable COPD. Modified from Ref. 11. SEMERGEN, SEPAR, semFYC, SEMG, SEFAC, GRAP. Referral criteria in COPD. Continuity of care. IMC 2023, Madrid. ISBN: 978-84-19457-41-7. Legal deposit: M-18163-2023.
* Consider interspersing telephone follow-up/nursing consultation with face-to-face consultations, especially in high-risk patients who are highly symptomatic and / or have frequent exacerbations.
Abbreviations: COPD: Chronic Obstructive Pulmonary Disease. ECG: electrocardiogram.
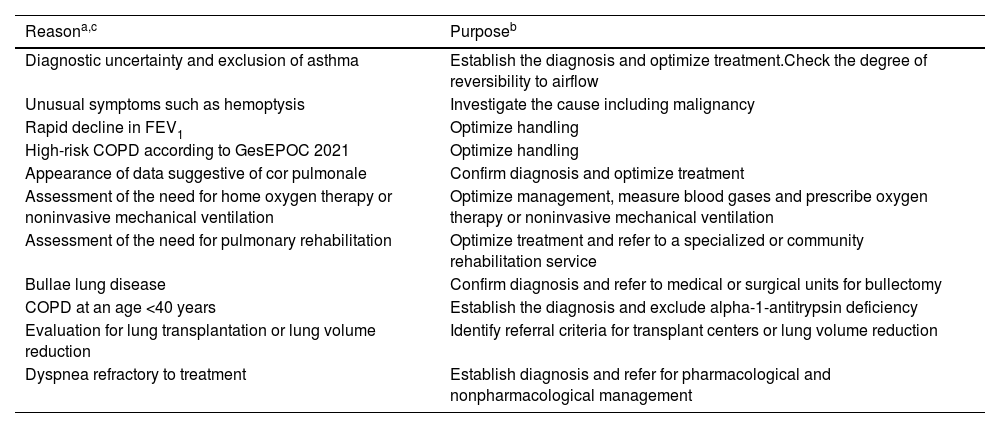
Regarding the indicators to refer a patient to the next level of care after the diagnosis of COPD, there is no standardization. The decision to refer depends not only on the clinical situation of the patient but also on other aspects, such as the experience of the health care professionals, the resources of the health center, the possibilities of follow-up and the distance from the home to the health center or hospital. Table 1 proposes a series of indicators that can help us in this regard.
Proposed criteria for referring COPD patients to pulmonology.
| Reasona,c | Purposeb |
|---|---|
| Diagnostic uncertainty and exclusion of asthma | Establish the diagnosis and optimize treatment.Check the degree of reversibility to airflow |
| Unusual symptoms such as hemoptysis | Investigate the cause including malignancy |
| Rapid decline in FEV1 | Optimize handling |
| High-risk COPD according to GesEPOC 2021 | Optimize handling |
| Appearance of data suggestive of cor pulmonale | Confirm diagnosis and optimize treatment |
| Assessment of the need for home oxygen therapy or noninvasive mechanical ventilation | Optimize management, measure blood gases and prescribe oxygen therapy or noninvasive mechanical ventilation |
| Assessment of the need for pulmonary rehabilitation | Optimize treatment and refer to a specialized or community rehabilitation service |
| Bullae lung disease | Confirm diagnosis and refer to medical or surgical units for bullectomy |
| COPD at an age <40 years | Establish the diagnosis and exclude alpha-1-antitrypsin deficiency |
| Evaluation for lung transplantation or lung volume reduction | Identify referral criteria for transplant centers or lung volume reduction |
| Dyspnea refractory to treatment | Establish diagnosis and refer for pharmacological and nonpharmacological management |
Abbreviations: COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in the first second; GesEPOC: Spanish COPD Guide.
The reason for referral is indicative of and will depend on the organizational structure of both levels of care where it is applied.
In the recently published document on referral criteria for patients with COPD,11 the proposed follow-up guidelines have been agreed upon by hospitals and primary care providers. We firmly believe that our proposal will be well received, both by the health professionals who care for these patients and by the hospital administrators responsible for establishing the necessary resources to guarantee adequate quality of care.
Authors’ contributionsEach author declares they have made an individual contribution to the article, all of them have materially participated in the research and/or preparation of the article.
ApprovalAll authors have authorized its publication.
FundingAll authors declare that there is no funding in this manuscript.
Conflict of interestJavier de Miguel Díez has received honoraria and funding from Laboratories AstraZeneca, Bial, Boehringer, Chiesi, Esteve, FAES, Ferrer, Gebro Pharma, GlaxoSmithKline, Janssen, Menarini, MundiPharma, Novartis, Roche, Rovi, Teva y Pfizer. Juan Marco Figueira Gonçalves has received honoraria for speaking engagements and funding for conference attendance from Laboratories Esteve, MundiPharma, AstraZeneca, Boehringer Ingelheim, Ferrer, Menarini, Rovi, GlaxoSmithKline, Chiesi, Novartis, and Gebro Pharma. Francisco Javier Plaza Zamora has received honoraria for speaking engagements and funding from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mundipharma, and Teva. María Sanz Almazán has received honoraria for speaking engagements and funding for conference attendance from Laboratories Menarini, GlaxoSmithKline, Vifor Pharma, AstraZeneca, Novo Nordisk, and Bial. Eva Trillo Calvo has received honoraria for speaking engagements and funding for conference attendance from Laboratories Menarini, GlaxoSmithKline, AstraZeneca, Lundbeck, Esteve, FAES, Boehringer Ingelheim, and Servier. Marta Villanueva Pérez has received honoraria for speaking engagements and funding from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Teva. Juan Enrique Cimas has received honoraria in the last three years for spirometry and COPD courses from semfyc and SESPA.