HIV can infect bronchial epithelial cells rendering individuals susceptible to lung damage. Our objective was to determine the effects of human immunodeficiency virus (HIV) infection on pulmonary function tests.
MethodsWe performed a meta-analysis after conducting a literature search in PubMed, Embase, Cochrane Library and Virtual Health Library databases from inception to December 31st, 2022. We employed the inverse variance method with a random effects model to calculate the effect estimate as the mean difference (MD) and 95% confidence interval (CI). We calculated the heterogeneity with the I2 statistic and performed a meta-regression analysis by age, sex, smoking, CD4 T-cells count and antiretroviral therapy. We also conducted a sensitivity analysis according to the studies’ publication date, and excluding the study with the greatest weight in the effect. The PROSPERO registry number was CRD42023401105.
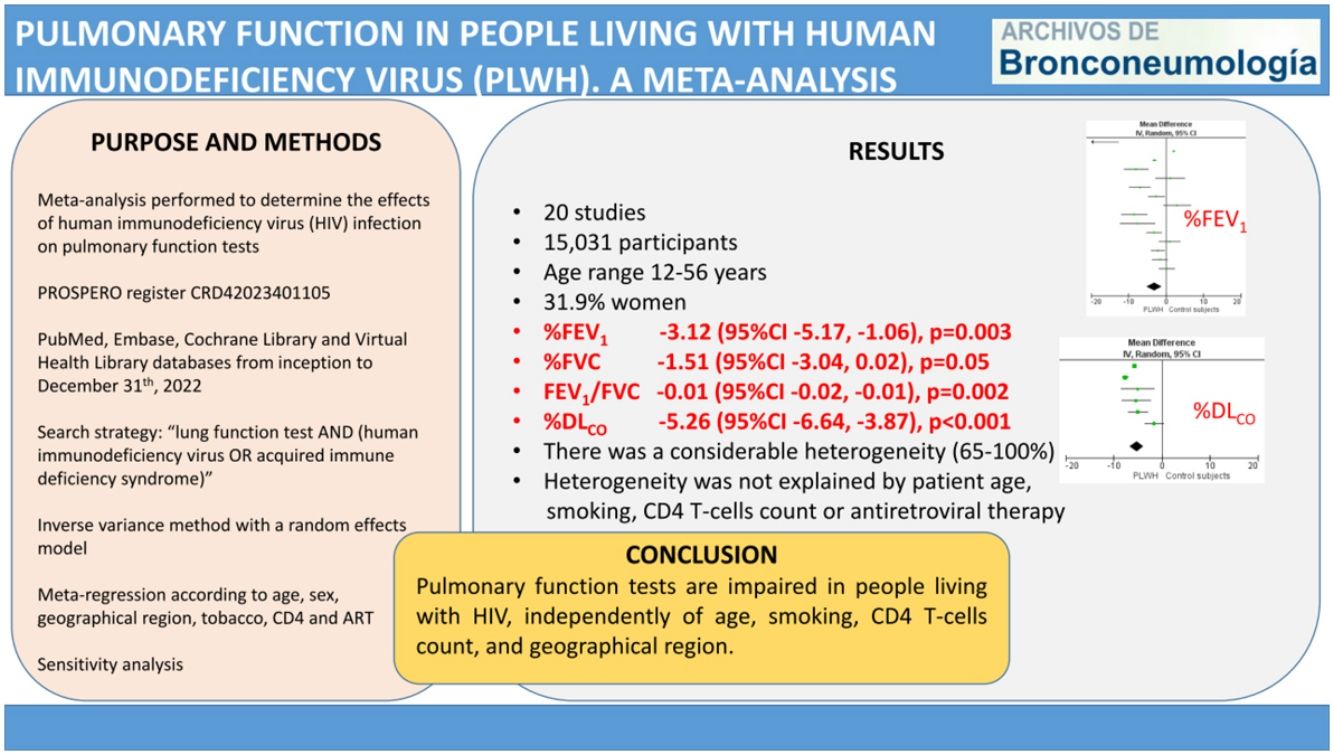
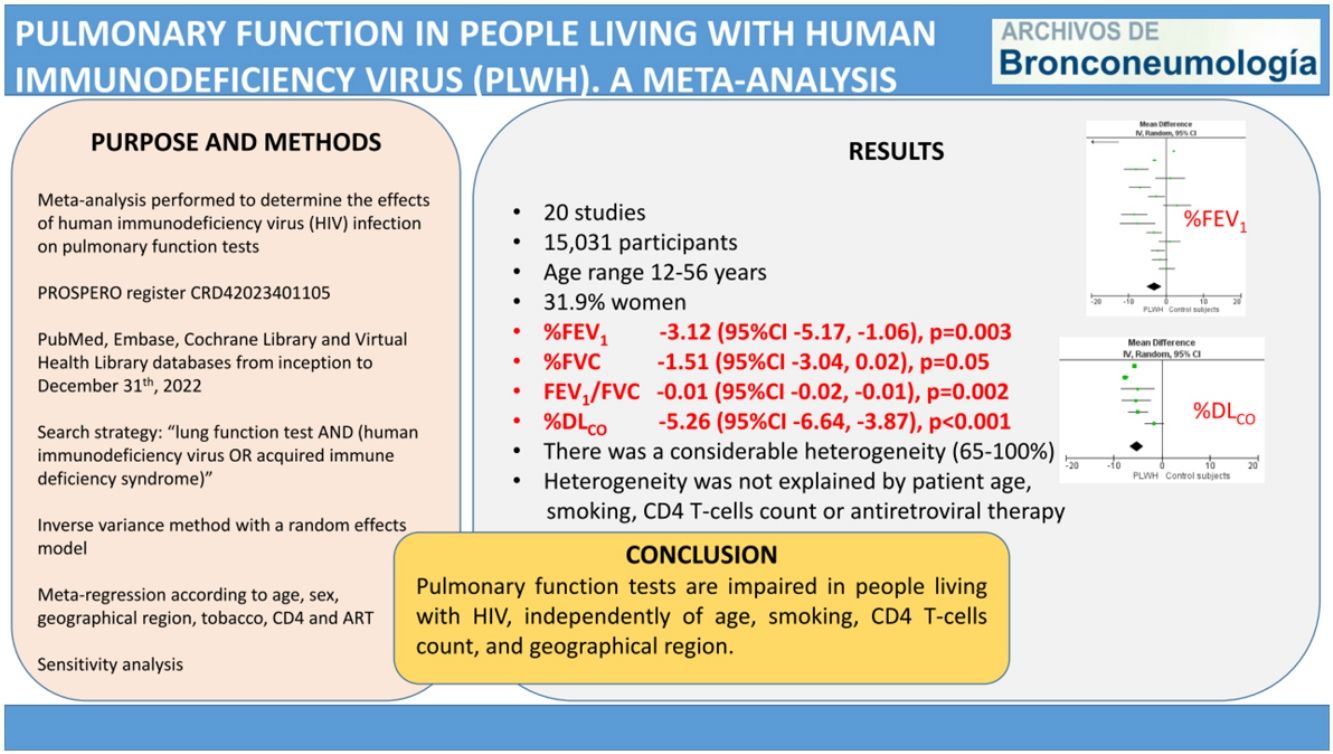
ResultsThe meta-analysis included 20 studies, with 7621 living with HIV and 7410 control participants. The pooled MD (95%CI) for the predicted percentage of FEV1, FVC and DLCO were −3.12 (−5.17, −1.06); p=0.003, −1.51 (−3.04, 0.02); p=0.05, and −5.26 (−6.64, −3.87); p<0.001, respectively. The pooled MD for FEV1/FVC was −0.01 (−0.02, −0.01); p=0.002. In all cases, there was a considerable heterogeneity. The meta-regression analysis showed that among studies heterogeneity was not explained by patient age, smoking, CD4 T-cells count or antiretroviral therapy.
ConclusionPulmonary function tests are impaired in people living with HIV, independently of age, smoking, CD4 T-cells count, and geographical region.
Human immunodeficiency virus (HIV) infection is a major health public problem worldwide. In 2021, it was estimated that 38.4 million people lived with HIV (PLWH), 1.5 million acquired HIV, and 650,000 died from HIV-related causes.1 HIV is a retrovirus that attacks the immune system causing a depletion of CD4 T lymphocytes, and favoring the appearance of opportunistic infections and neoplasms.
It has been suggested that the lung might serve as an anatomical HIV reservoir.2 HIV can infect bronchial epithelial cells rendering individuals susceptible to lung damage. HIV can also infect alveolar macrophages impairing phagocytosis and immune response to pathogens, and leading to HIV persistence in lungs.3
From detection of the first case of acquired immunodeficiency syndrome in 1982, Pneumocysis jirovecii pneumonia, tuberculosis and other lung infections were frequent in PLWH.4 Later, several studies have reported that lung diseases as airflow limitation, emphysema and respiratory infections are associated with HIV infection.5–7 In the combination antiretroviral therapy era, comorbid lung diseases are important determinants of morbidity and mortality in PLWH.8 A recent meta-analysis estimated a prevalence of chronic obstructive pulmonary disease (COPD) of approximately 10.6% in PLWH, and a multicenter cohort study observed that PLWH have faster lung function decline than matched population controls.9,10 However, research on lung function in PLWH has shown controversial results.
Therefore, we performed a meta-analysis to investigate the effects of HIV infection on pulmonary function comparing lung function tests between PLWH and uninfected controls.
Study design and methodsWe designed this meta-analysis to determine, in PLWH, the changes on the following parameters of pulmonary function test: forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and diffusion capacity of the lung for carbon monoxide (DLCO).
We recorded the protocol for this meta-analysis in the PROSPERO registry (number CRD42023401105) and conducted according to the guidelines of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group.
Data sources and search strategyWe performed a systematic search in PubMed, EMBASE, the Cochrane Library and Virtual Health Library databases from their inception to December 31st, 2022. The search strategy in PubMed, the Cochrane Library and the Virtual Health Library was “lung function test AND (human immunodeficiency virus OR acquired immune deficiency syndrome)” and in EMBASE “(‘lung function test’/exp OR ‘lung function test’) AND (‘human immunodeficiency virus’/exp OR ‘human immunodeficiency virus’ OR ‘acquired immune deficiency syndrome’/exp OR ‘acquired immune deficiency syndrome’)”. We performed an additional search in ResearchGate and GoogleScholar, and screened manually the reference lists of the selected articles to find more studies.
Study selection and quality assessmentTo be included in this review, the studies had to meet the following inclusion criteria:
- (i)
Presence of a PLWH group and a control group without HIV infection.
- (ii)
Provide values either of FEV1, FVC, DLCO and/or FEV1/FVC ratio for both groups.
The exclusion criteria were studies including patients with active tuberculosis, studies that did not report data on mean and standard deviation or its calculation was not possible. When two studies referred to the same population, in the same period and showed overlapping data, we selected the most recent study for inclusion. There was no language restriction.
Both authors reviewed the titles and abstracts of the articles, and recovered the studies that met the inclusion criteria and those with abstracts that lacked crucial information to evaluate the full text. Any disagreement was resolved by consensus.
We independently assessed the risk of bias of all the studies included using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (National Heart, Lung, and Blood Institute at the National Institutes of Health, USA), available from https://www.nhlbi.nih.goov/health-topics/study-quality-assessment-tools.
Data synthesis and statistical analysisFrom each included study, we extracted the following information; first author, year of publication, country, sample size, age, sex, tobacco use, mean/median CD4 (cell/μL), and use of antiretroviral therapy. The extracted results were FEV1 (liters, L), percentage of predicted (%) FEV1, FVC (L), %FVC, FEV1/FVC ratio, %DLCO, DLCO (mL/min/mmHg). Whenever the PLWH or control group were divided into subgroups, a pooled mean and standard deviation for these combined subgroups was calculated. When studies reported data as median and interquartile range, we calculated the mean and standard deviation with the Wan et al. method.11
We performed the statistical analysis using Review Manager version 5.4.1 (Cochrane Collaboration, Baltimore, Maryland, USA) and Open/Meta[Analyst] (Brown University). The results are expressed as mean differences (MD) with 95% confidence intervals (CI). Throughout the analysis, we applied the inverse variance method with a random effects model. We employed the tau squared and I squared (I2) statistics to assess the heterogeneity and inconsistency among the studies. Data with p≥0.10 and I2≤50% were defined as low heterogeneity. We evaluated the publication bias with a funnel plot. We planned a subgroup analysis and meta-regression according to age, sex, geographical region, tobacco use, CD4 T-cells count and antiretroviral therapy. We performed a sensitivity analysis by calculating the effect estimates according to publication date, and by eliminating conference proceedings and the study with the greatest weight on the effect. We established two categories of publication year, before and after year 1996, year of introduction of the high intensity antiretroviral therapy.
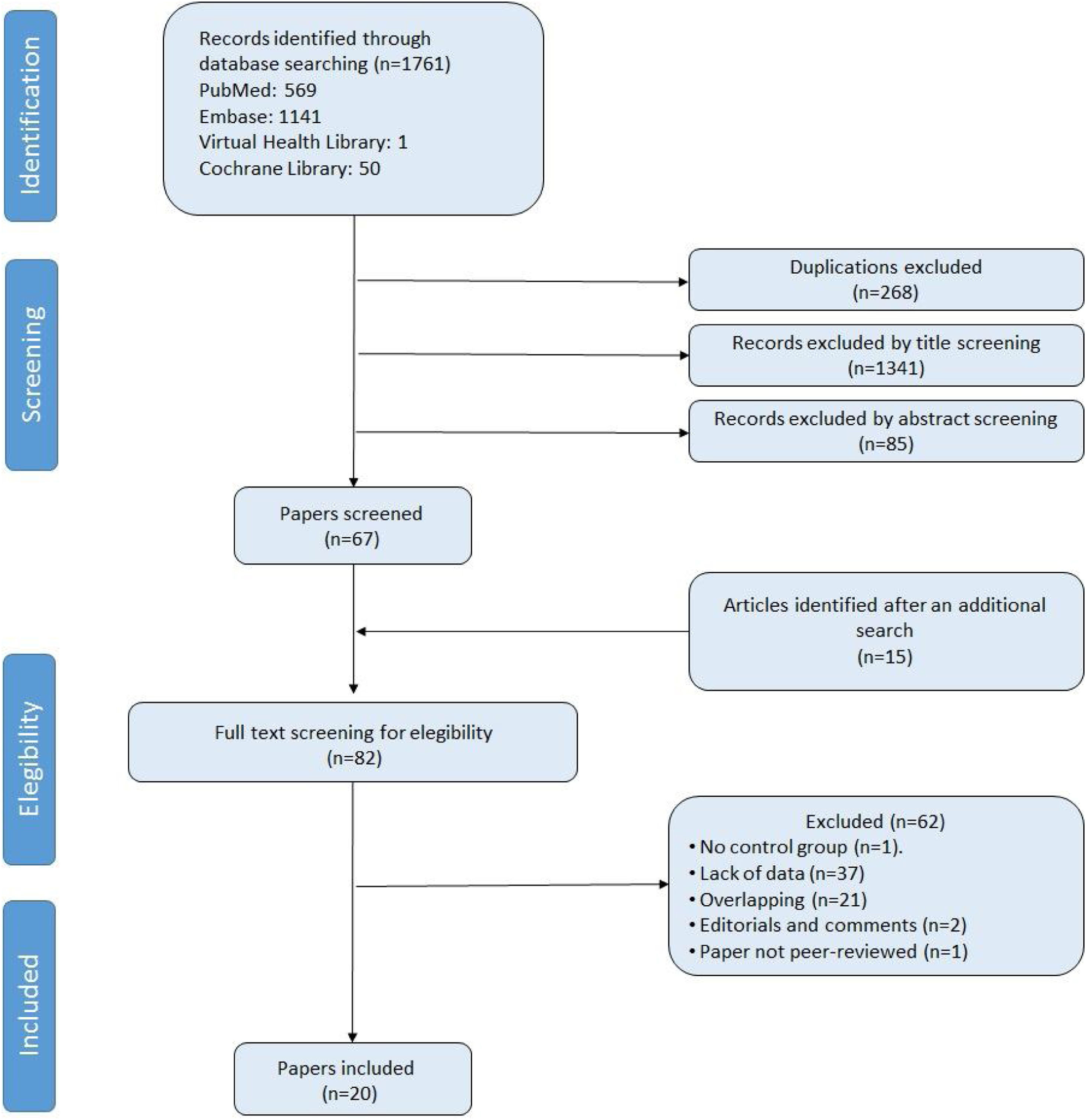
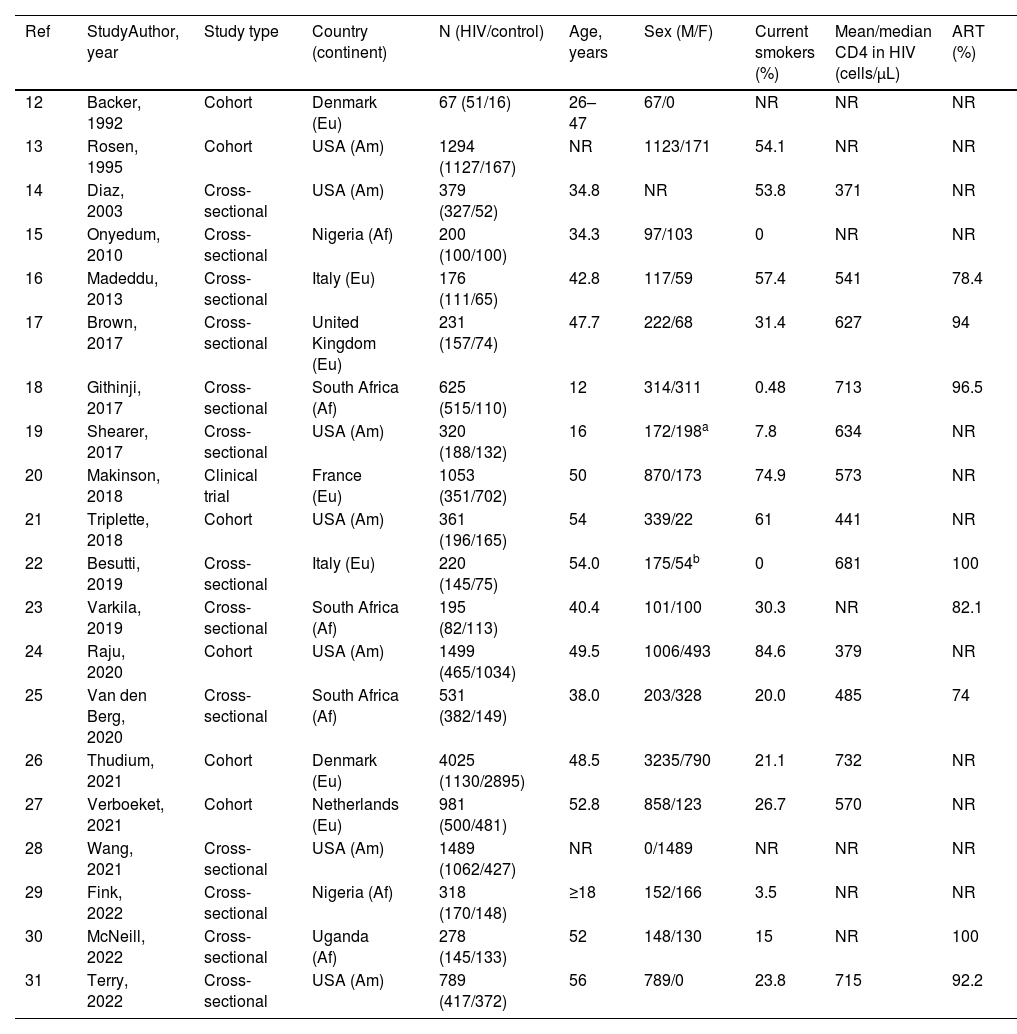
ResultsStudy selection and characteristicsWe identified 1776 records. Fig. 1 shows the study selection flowchart. Our initial search strategy produced 1761 articles. With the manual search of the reference lists and the additional search in GoogleScholar and ResearchGate, we added 15 papers. After eliminating the duplicated and irrelevant articles, we were left with 82 articles. We excluded 62 articles for the following reasons: one had no control group, 37 provided insufficient numerical data to be included in the meta-analysis, 21 presented overlapping data, two were comments and editorials, and one was a posted not peer-reviewed draft. Ultimately, we included 20 studies in the meta-analysis,12–31 a clinical trial, 6 cohort studies and 13 cross-sectional ones.
We report the characteristics of the included studies in Table 1. They were published between 1992 and 2022. All studies were written in English, and after the quality assessment, were classified as good. The inter-rater agreement was full. A total of 15,031 participants were included, 7621 in the PLWH group and 7410 in the control group. The age range of participants was 12–56 years, and 31.9% were women.
Characteristics of the included studies.
| Ref | StudyAuthor, year | Study type | Country (continent) | N (HIV/control) | Age, years | Sex (M/F) | Current smokers (%) | Mean/median CD4 in HIV (cells/μL) | ART (%) |
|---|---|---|---|---|---|---|---|---|---|
| 12 | Backer, 1992 | Cohort | Denmark (Eu) | 67 (51/16) | 26–47 | 67/0 | NR | NR | NR |
| 13 | Rosen, 1995 | Cohort | USA (Am) | 1294 (1127/167) | NR | 1123/171 | 54.1 | NR | NR |
| 14 | Diaz, 2003 | Cross-sectional | USA (Am) | 379 (327/52) | 34.8 | NR | 53.8 | 371 | NR |
| 15 | Onyedum, 2010 | Cross-sectional | Nigeria (Af) | 200 (100/100) | 34.3 | 97/103 | 0 | NR | NR |
| 16 | Madeddu, 2013 | Cross-sectional | Italy (Eu) | 176 (111/65) | 42.8 | 117/59 | 57.4 | 541 | 78.4 |
| 17 | Brown, 2017 | Cross-sectional | United Kingdom (Eu) | 231 (157/74) | 47.7 | 222/68 | 31.4 | 627 | 94 |
| 18 | Githinji, 2017 | Cross-sectional | South Africa (Af) | 625 (515/110) | 12 | 314/311 | 0.48 | 713 | 96.5 |
| 19 | Shearer, 2017 | Cross-sectional | USA (Am) | 320 (188/132) | 16 | 172/198a | 7.8 | 634 | NR |
| 20 | Makinson, 2018 | Clinical trial | France (Eu) | 1053 (351/702) | 50 | 870/173 | 74.9 | 573 | NR |
| 21 | Triplette, 2018 | Cohort | USA (Am) | 361 (196/165) | 54 | 339/22 | 61 | 441 | NR |
| 22 | Besutti, 2019 | Cross-sectional | Italy (Eu) | 220 (145/75) | 54.0 | 175/54b | 0 | 681 | 100 |
| 23 | Varkila, 2019 | Cross-sectional | South Africa (Af) | 195 (82/113) | 40.4 | 101/100 | 30.3 | NR | 82.1 |
| 24 | Raju, 2020 | Cohort | USA (Am) | 1499 (465/1034) | 49.5 | 1006/493 | 84.6 | 379 | NR |
| 25 | Van den Berg, 2020 | Cross-sectional | South Africa (Af) | 531 (382/149) | 38.0 | 203/328 | 20.0 | 485 | 74 |
| 26 | Thudium, 2021 | Cohort | Denmark (Eu) | 4025 (1130/2895) | 48.5 | 3235/790 | 21.1 | 732 | NR |
| 27 | Verboeket, 2021 | Cohort | Netherlands (Eu) | 981 (500/481) | 52.8 | 858/123 | 26.7 | 570 | NR |
| 28 | Wang, 2021 | Cross-sectional | USA (Am) | 1489 (1062/427) | NR | 0/1489 | NR | NR | NR |
| 29 | Fink, 2022 | Cross-sectional | Nigeria (Af) | 318 (170/148) | ≥18 | 152/166 | 3.5 | NR | NR |
| 30 | McNeill, 2022 | Cross-sectional | Uganda (Af) | 278 (145/133) | 52 | 148/130 | 15 | NR | 100 |
| 31 | Terry, 2022 | Cross-sectional | USA (Am) | 789 (417/372) | 56 | 789/0 | 23.8 | 715 | 92.2 |
Af: Africa; Am: America; ART: antiretroviral therapy; Eu: Europe; HIV: human immunodeficiency virus; Ref: reference; USA: United States of America.
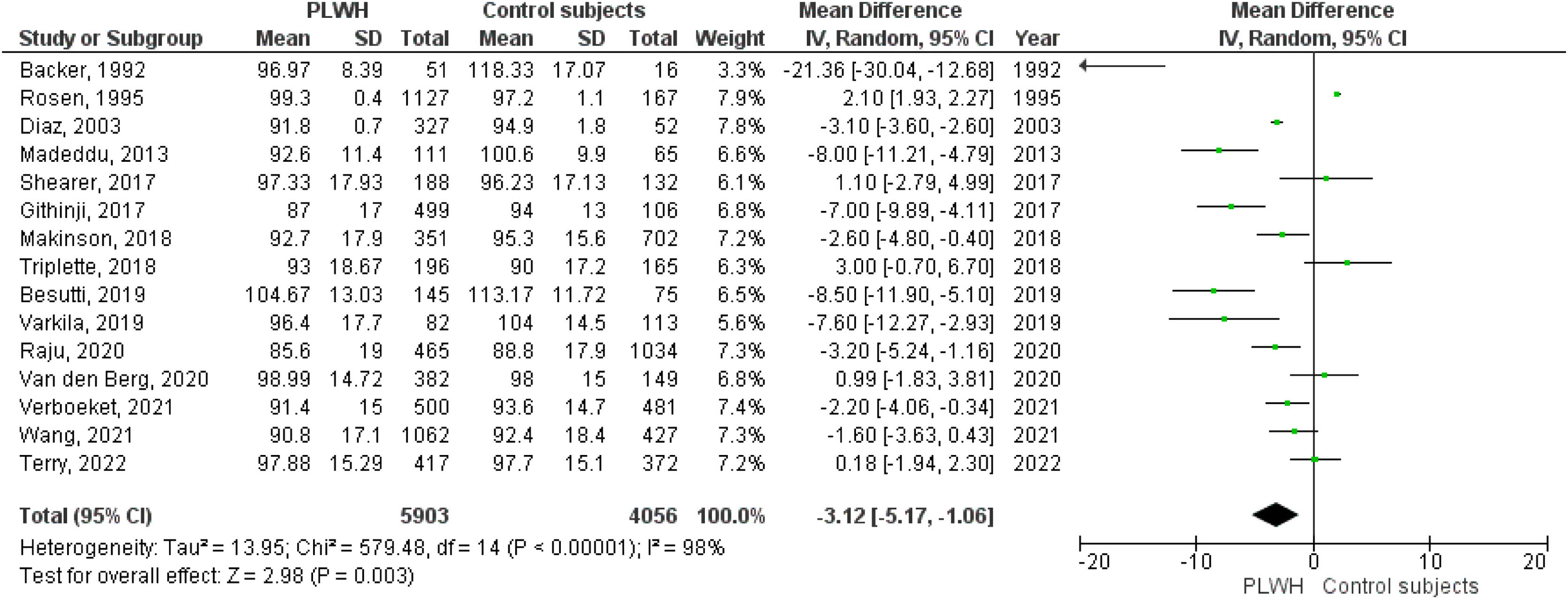
A total of 15 studies included data on %FEV1, and 12 included data on FEV1(L). The pooled effect estimates for PLWH were −3.12 (95%CI −5.17 to −1.06; p=0.003) for %FEV1 and −0.19 (95%CI −0.29 to −0.06; p<0.001) for FEV1(L).
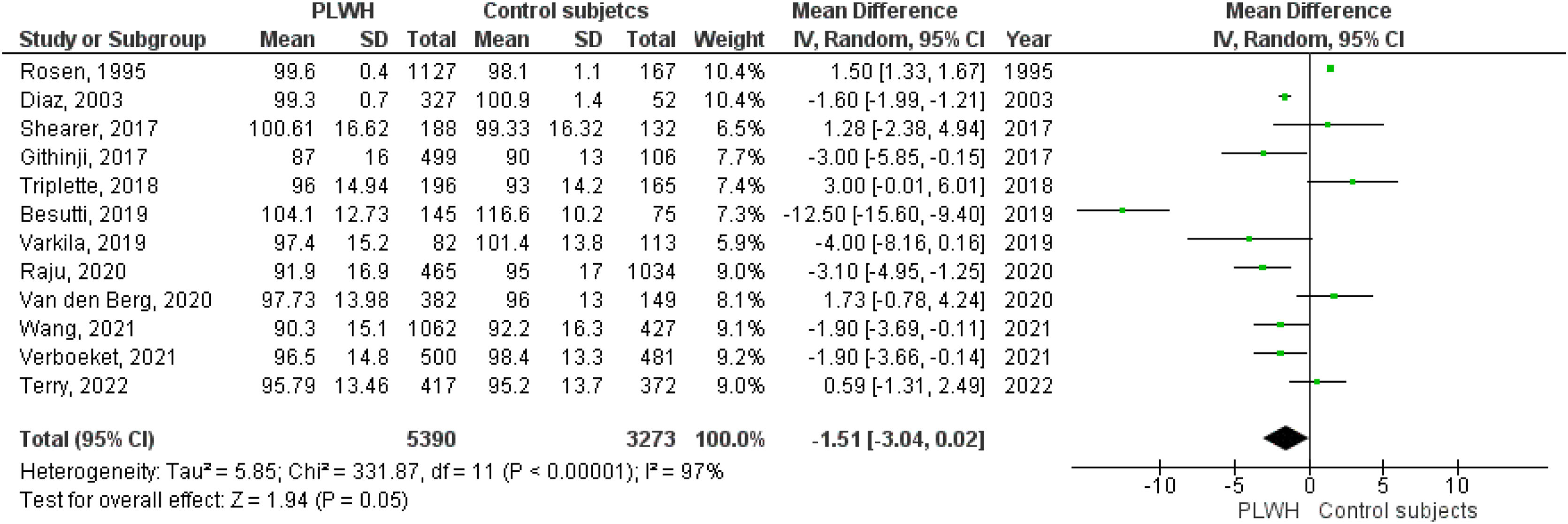
Twelve studies included data on %FVC, and eleven included data on FVC(L). The pooled estimates for PLWH were −1.51 (95%CI −3.04 to 0.02; p=0.05) for %FVC and −0.17 (95%CI −0.26 to −008; p<0.001) for FVC(L).
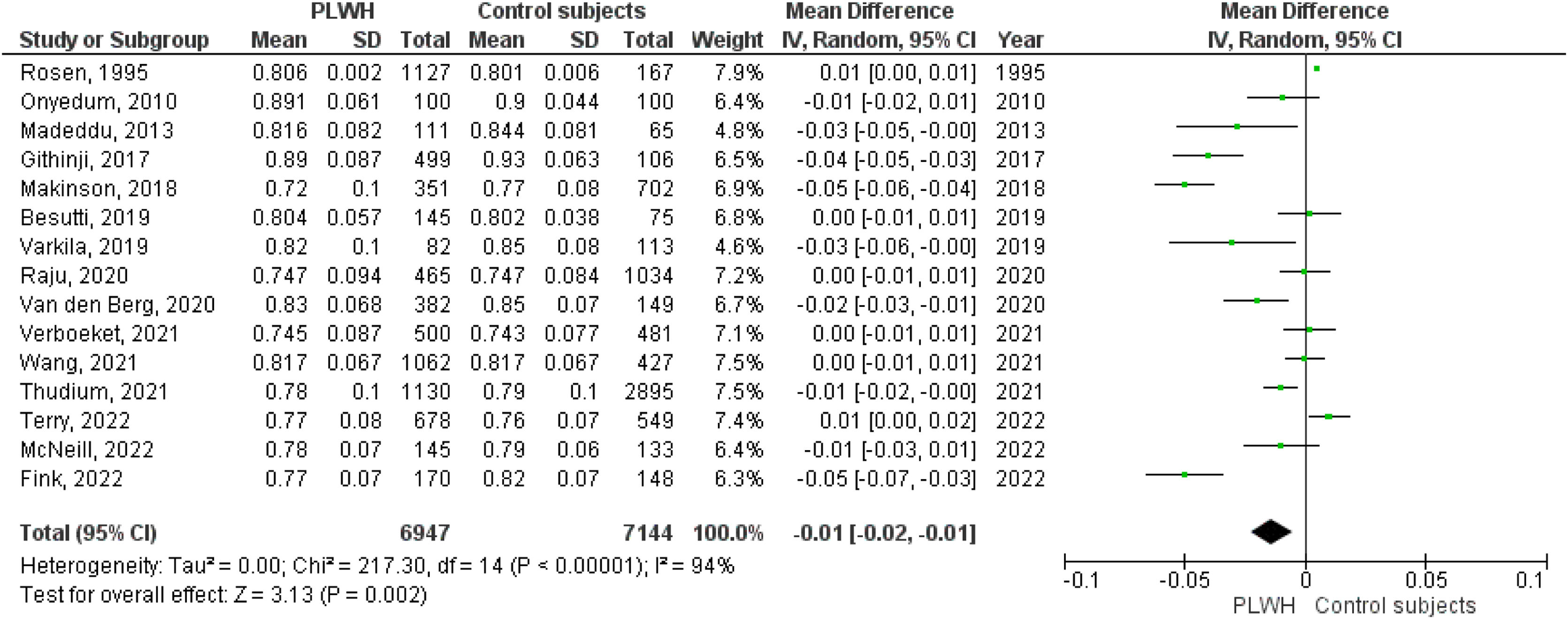
A total of 15 studies included data on the FEV1/FVC ratio. The pooled effect estimate for PLWH was −0.01 (95%CI −0.02 to −0.01; p=0.002).
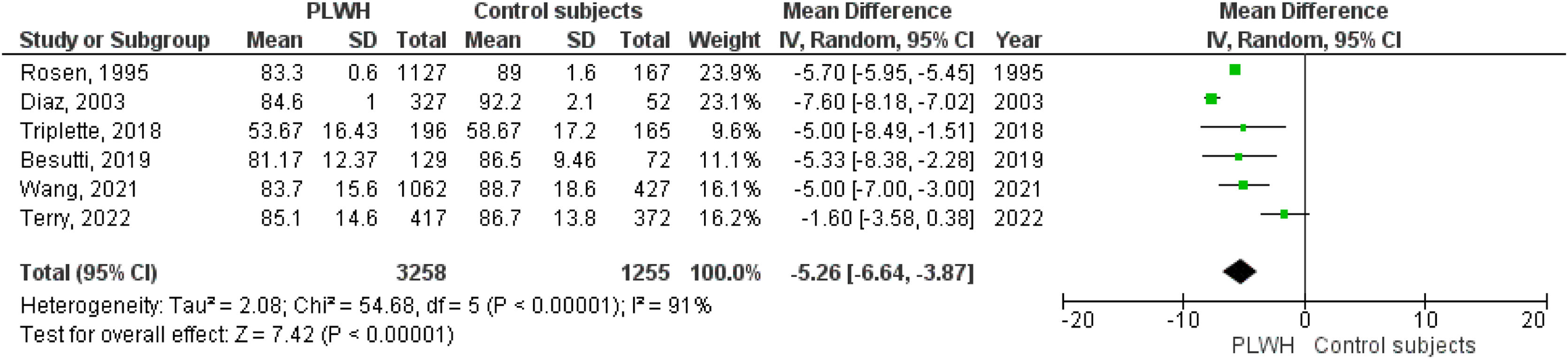
Six studies included data on %DLCO, and three included data on DLCO (mL/min/mmHg). The pooled effect estimates for PLWH were −5.26 (95%CI −6.64 to −3.87; p<0.001) for %DLCO and −1.78 (95%CI −1.87 to −1.69; p<0.001 for DLCO (mL/min/mmHg).
Figs. 2–5 show the comparison forest plots for %FEV1, %FVC, FEV1/FVC ratio, and %DLCO, and e-Figs. 1–3 for FEV1(L), FVC(L) and DLCO (mL/min/mmHg), respectively. There was significant heterogeneity for all parameters of the pulmonary function tests (I2 65–100%).
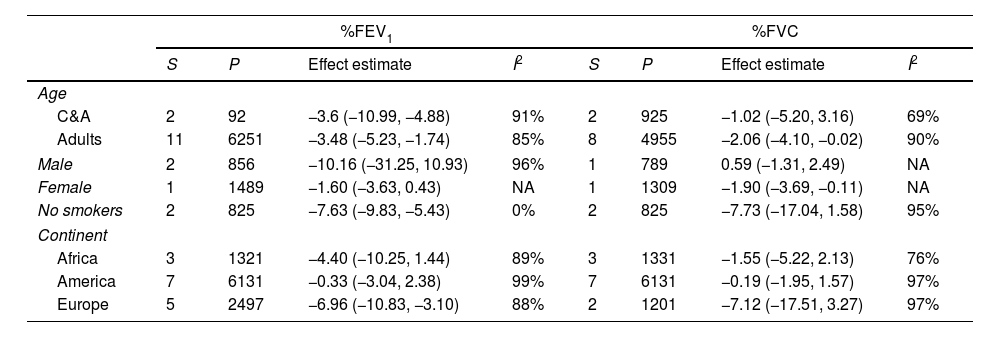
Subgroup analysisTable 2 present the pre-specified subgroup analysis for %FEV1, %FVC, FEV1/FVC ratio, and %DLCO, and e-Table 1 for FEV1(L), FVC(L) and DLCO (mL/min/mmHg), respectively. Sixteen studies were conducted in adults and two in children and adolescents. When we excluded from analysis the studies performed in children and adolescents the impairment of pulmonary function tests was similar. Two studies included exclusively men and one women. Another study reported data differentiated by sex. However, we cannot perform a meta-analysis.
Subgroup analysis.
| %FEV1 | %FVC | |||||||
|---|---|---|---|---|---|---|---|---|
| S | P | Effect estimate | I2 | S | P | Effect estimate | I2 | |
| Age | ||||||||
| C&A | 2 | 92 | −3.6 (−10.99, −4.88) | 91% | 2 | 925 | −1.02 (−5.20, 3.16) | 69% |
| Adults | 11 | 6251 | −3.48 (−5.23, −1.74) | 85% | 8 | 4955 | −2.06 (−4.10, −0.02) | 90% |
| Male | 2 | 856 | −10.16 (−31.25, 10.93) | 96% | 1 | 789 | 0.59 (−1.31, 2.49) | NA |
| Female | 1 | 1489 | −1.60 (−3.63, 0.43) | NA | 1 | 1309 | −1.90 (−3.69, −0.11) | NA |
| No smokers | 2 | 825 | −7.63 (−9.83, −5.43) | 0% | 2 | 825 | −7.73 (−17.04, 1.58) | 95% |
| Continent | ||||||||
| Africa | 3 | 1321 | −4.40 (−10.25, 1.44) | 89% | 3 | 1331 | −1.55 (−5.22, 2.13) | 76% |
| America | 7 | 6131 | −0.33 (−3.04, 2.38) | 99% | 7 | 6131 | −0.19 (−1.95, 1.57) | 97% |
| Europe | 5 | 2497 | −6.96 (−10.83, −3.10) | 88% | 2 | 1201 | −7.12 (−17.51, 3.27) | 97% |
| FEV1/FVC ratio | % DLCO | |||||||
|---|---|---|---|---|---|---|---|---|
| S | P | Effect estimate | I2 | S | P | Effect estimate | I2 | |
| Age | ||||||||
| C&A | 1 | 605 | −0.04 (−0.05, −0.03) | NA | 0 | 0 | – | – |
| Adults | 12 | 10,703 | −0.02 (−0.03, 0.00) | 90% | 5 | 3024 | −5.29 (−6.85, −3.73) | 93% |
| Male | 1 | 97 | −0.02 (−0.04, 0.01) | NA | 1 | 789 | −1.60 (−3.58, 0.38) | NA |
| Female | 2 | 1592 | 0%- | 1 | 1489 | −5.00 (−7.00, −3.00) | NA | |
| No smokers | 3 | 1025 | −0.02 (−0.04, 0.01) | 90% | 1 | 201 | −5.33 (−8.38, −2.28) | NA |
| Continent | ||||||||
| Africa | 6 | 2127 | −0.03 (−0.04, −0.01) | 78% | 0 | 0 | – | – |
| America | 3 | 4282 | 0.00 (0.00, 0.01) | 23% | 5 | 3494 | −5.24 (−6.70, −3.83) | 93% |
| Europe | 5 | 6455 | −0.02 (−0.03, 0.01) | 90% | 1 | 201 | −5.33 (−8.38, −2.28) | NA |
C&A: children and adolescents; DLCO: diffusion capacity of the lung for carbon monoxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; NA: not applicable; P: participants; S: studies.
Sixteen studies included patients who smoked and those who did not, and two studies included exclusively nonsmokers. Another two studies did not report data on tobacco use. In one study, the prevalence of smoking was 0.48% and we considered it as nonsmoking in the analysis. There was heterogeneity between the groups; the effect estimate for PLWH who did not smoke presented a reduction in %FEV1, FEV1(L), and FVC(L), p≤0.01 for all, which was higher than in the other studies than included smokers and nonsmokers. However, there was a scarce number of studies in nonsmokers and with a limited number of participants.
Six studies were conducted in Africa, 7 in America and 7 in Europe. We observed a high heterogeneity among the various continents in all pulmonary function tests.
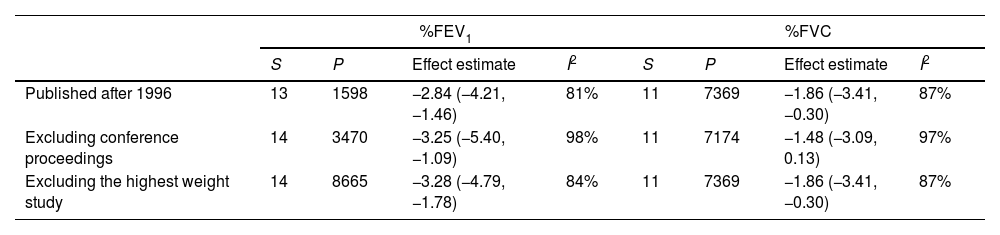
Sensitivity analysis and meta-regressionWhen we performed an analysis separated by publication year, before and after 1996, we observed the same abnormal pulmonary function test results. The same result occurred when we excluded the proceedings of congresses (Table 3 for %FEV1, %FVC, FEV1/FVC ratio and %DLCO, and e-table 2 for FEV1(L), FVC(L) and DLCO (mL/min/mmHg)). The removal of the study with the greatest weight in each pulmonary function test did not change the results.
Sensitivity analysis.
| %FEV1 | %FVC | |||||||
|---|---|---|---|---|---|---|---|---|
| S | P | Effect estimate | I2 | S | P | Effect estimate | I2 | |
| Published after 1996 | 13 | 1598 | −2.84 (−4.21, −1.46) | 81% | 11 | 7369 | −1.86 (−3.41, −0.30) | 87% |
| Excluding conference proceedings | 14 | 3470 | −3.25 (−5.40, −1.09) | 98% | 11 | 7174 | −1.48 (−3.09, 0.13) | 97% |
| Excluding the highest weight study | 14 | 8665 | −3.28 (−4.79, −1.78) | 84% | 11 | 7369 | −1.86 (−3.41, −0.30) | 87% |
| FEV1/FVC ratio | % DLCO | |||||||
|---|---|---|---|---|---|---|---|---|
| S | P | Effect estimate | I2 | S | P | Effect estimate | I2 | |
| Published after 1996 | 14 | 12,797 | −0.02 (−0.03, −0.01) | 90% | 5 | 3219 | −4.96 (−7.56, −2.35) | 90% |
| Excluding conference proceedings | 13 | 8577 | −0.02 (−0.03, −0.00) | 94% | 5 | 3024 | −5.29 (−6.85, −3.73) | 93% |
| Excluding the highest weight study | 14 | 12,979 | −0.02 (−0.03, −0.01) | 90% | 5 | 3219 | −4.96 (−7.56, −3.35) | 90% |
DLCO: diffusion capacity of the lung for carbon monoxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; P: participants; S: studies.
The meta-regression analysis showed that the heterogeneity among the studies was not explained by patient age, smoking, CD4 T-cells count or antiretroviral therapy (e-Table 3).
Publication biasThe funnel plots (e-Fig. 4) showed asymmetry, indicating the presence of potential publication bias.
DiscussionThe results of our meta-analysis show that all pulmonary function tests were decreased in PLWH. This pulmonary function impairment in PLWH is also observed in nonsmokers and is independent of age, CD4 T-cells count and antiretroviral therapy.
To our knowledge, this is the first meta-analysis on pulmonary function in PLWH. A previously published meta-analysis has focused on prevalence of COPD in PLWH.9 It included 30 studies with 151,686 participants and the overall prevalence of COPD was higher in PLWH than in uninfected controls. Tobacco is a leading cause of lung damage. Worldwide, smoking prevalence among PLWH is higher than in HIV uninfected population.32 Peculiarly, in our meta-analysis, the impairment of pulmonary function remained when we included only those studies with nonsmoker patients.
It is widely known that PLWH have more pulmonary diseases and infections, including pneumonia and tuberculosis.8 The lung is a reservoir for HIV. DNA and RNA of HIV are present in alveolar macrophages in asymptomatic healthy PLWH with systemic viral suppression.33 Besides, there are structural abnormalities in the lung of PLWH that could explain the abnormal pulmonary function test results. Lymphocytic alveolitis, with infiltration of CD4+ and CD8+ T-cells, gamma-delta T-cells and B-cells, is present in untreated PLWH.34 Computed tomographic examinations have detected emphysema and fibrosis-like changes in PLWH that were significantly correlated with HIV viral load.35 Interestingly, one study has shown a telomere shorthening of small airway epithelial cells.36 Inflammation and accelerated immune aging are both proposed to contribute to lung impairment in PLWH.
A decrease in the FEV1/FVC ratio suggests that HIV may be a risk factor for airflow obstruction. This hypothesis is supported by the findings of a meta-analysis by Bigna et al., which found that the prevalence of COPD is higher in PLWH than in the general population.9 While a decrease in FEV1 indicates the presence of obstruction and a decrease in FVC indicates restriction, the simultaneous decline of both parameters could indicate an isolated restrictive pattern or a combined obstructive and restrictive pattern.27 The presence of emphysema and fibrosis observed on computed tomography or lymphocytic alveolitis in lung biopsies of PLWH could explain these alterations. Alveolitis and fibrosis-like changes observed in lungs of PLWH can induce interstitial and vascular abnormalities that compromise the gas exchange. This would manifest as a reduction in DLCO. It has been suggested that HIV-induced inflammation could damage the small airways.27 However, we did not find sufficient data on FEF25–75% to be included in this meta-analysis.
Various immune and biochemical mechanisms have been proposed to explain the pulmonary damage observed in PLWH.2,37 Alexandrova et al. propose that pulmonary immune dysregulation maintains a proinflammatory environment optimal for HIV reservoir persistence.2 Head et al., in a systematic review, described nine immune biomarkers with significant association with lung abnormality, including interleukin-6, tumor necrosis factor α, C-reactive protein, cathelicidin, soluble CD14, soluble CD163, interleukin-2 receptor α chain, endothelin-1, and CD4/CD8 ratio.37 These findings suggest that inflammation, innate immune system, monocyte activation, endothelial dysfunction and general immune health could play a role in HIV-related pulmonary impairment.
The functional impairment observed in PLWH for FEV1 and FVC appear modest but is approximately 180–190mL. Similar differences, 100–150mL, have been considered significant in clinical trials with bronchodilators agents in patients with COPD.38 Therefore, pulmonary function impairment in PLWH is relevant. Longitudinal studies have reported a reduction of DLCO over time, and recently, distinct longitudinal pulmonary function trajectories have been identified.39,40 However, we think that prospective longitudinal studies are still needed to elucidate the progression of pulmonary impairment in PLWH.
Heterogeneity is an important finding in our meta-analysis. There are several possible reasons for it. Firstly, there are differences in participants of studies. The mean age of individuals ranged from 12 to 56 years, the proportion of male included from 0 to 100%, the mean/median CD4 cell/μL count from 371 to 732 and 52–100% PLWH toke antiretroviral therapy. Even there are differences in ethnicity of patients included in various studies conducted in United States. Secondly, we cannot rule out the influence of diverseness in lung function measurement methodology, i.e. equipment and reference equations. Also, the effect estimates of absolute lung function values not stratified by sex and age need to be interpreted with caution. Finally, the funnel plots were asymmetric suggesting a possible publication bias. The existence of not published small studies is the most frequent cause of asymmetry in a funnel plot.
The lack of data on viremia and duration of HIV infection, and the scarce data about CD4 T-cells count and antiretroviral therapy are limitations of this study and the results of meta-regression should therefore be taken with caution and should be validated in future studies. In addition, surprisingly, we have not identified any study from Asian and south American countries.
However, our study has some strengths. We applied the recommended MOOSE guideline for meta-analysis of observational studies. The literature research was exhaustive and comprehensive without language restrictions. We performed a sensitivity analysis, and the pulmonary function test impairment in PLWH were maintained when we differentiated them by study publication year, when we excluded gray literature, and even when we excluded the study with the greatest weight, all of which reinforces the results of the meta-analysis.
In conclusion, HIV infection is associated with pulmonary function impairment. However, further studies from all geographical areas are needed to corroborate these findings, and it is recommendable to include the pulmonary function tests as an additional outcome in clinical trials of new drugs for HIV infection.
FundingNone.
Conflict of interestThe authors declare no conflict of interest
Jesús Díez-Manglano and Esther Del Corral Beamonte participated in study design, literature search, data collection, data analysis, and data interpretation. Jesús Díez-Manglano drafted the manuscript, and Esther Del Corral Beamonte contributed and approved the final version of the manuscript.