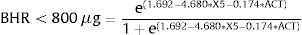Bronchial hyperresponsiveness (BHR) is a hallmark of asthma and a useful tool for the diagnosis and evaluation of disease control.1 However, bronchial provocation is a time-consuming procedure, usually limited to specific centers.2 Moreover, the use of nebulized medication further reduced its implementation during the COVID-19 pandemic. BHR has been linked to asthma control3 and has long been associated with the presence of inflammation in the small airways of asthmatic patients.4 Several parameters of small airways disease (SAD) in association with fractional exhaled nitric oxide (FeNO) as marker of inflammation have been used in different populations of patients with asthma to detect or predict BHR, with different results.5–7 The aim of the present study was to investigate the relationship between BHR, asthma control and SAD parameters in patients with mild/moderate asthma exploring the possibility of predicting BHR.
Outpatients with asthma were evaluated with spirometry, static lung volumes, single breath nitrogen washout, (FeNO) and impulse oscillometry [Jaeger Masterscope CT IOS (CareFusion, Hoechberg, Germany)]. All patients were under treatment with combination of long acting beta2 agonists and inhaled corticosteroids, without an exacerbation during the last four weeks; 19% were smokers with a smoking history <10 pack-years. Patient demographics are presented in Table 1. Asthma control was evaluated with the asthma control test (ACT) and the asthma control questionnaire 6-item (ACQ-6), and BHR with methacholine bronchial challenge using a Jaeger APS dosimeter. Criteria for SAD diagnosis were as follows: FEF25–75≤60% pred., RV/TLC>35% pred., RV>120% pred., FRC>120% pred., N2Delta>1.5%, R5-R20>0.07kPa/L/s, Frs>15 and AX>0.8.8 Well-controlled asthma was defined by values of ACT>20 or ACQ<1.5 and BHR was defined as the provocative dose of methacholine producing 20% fall in FEV1 and was detected if PD20<800μg. All patients provided written consent and the study was approved by the hospital's scientific committee.
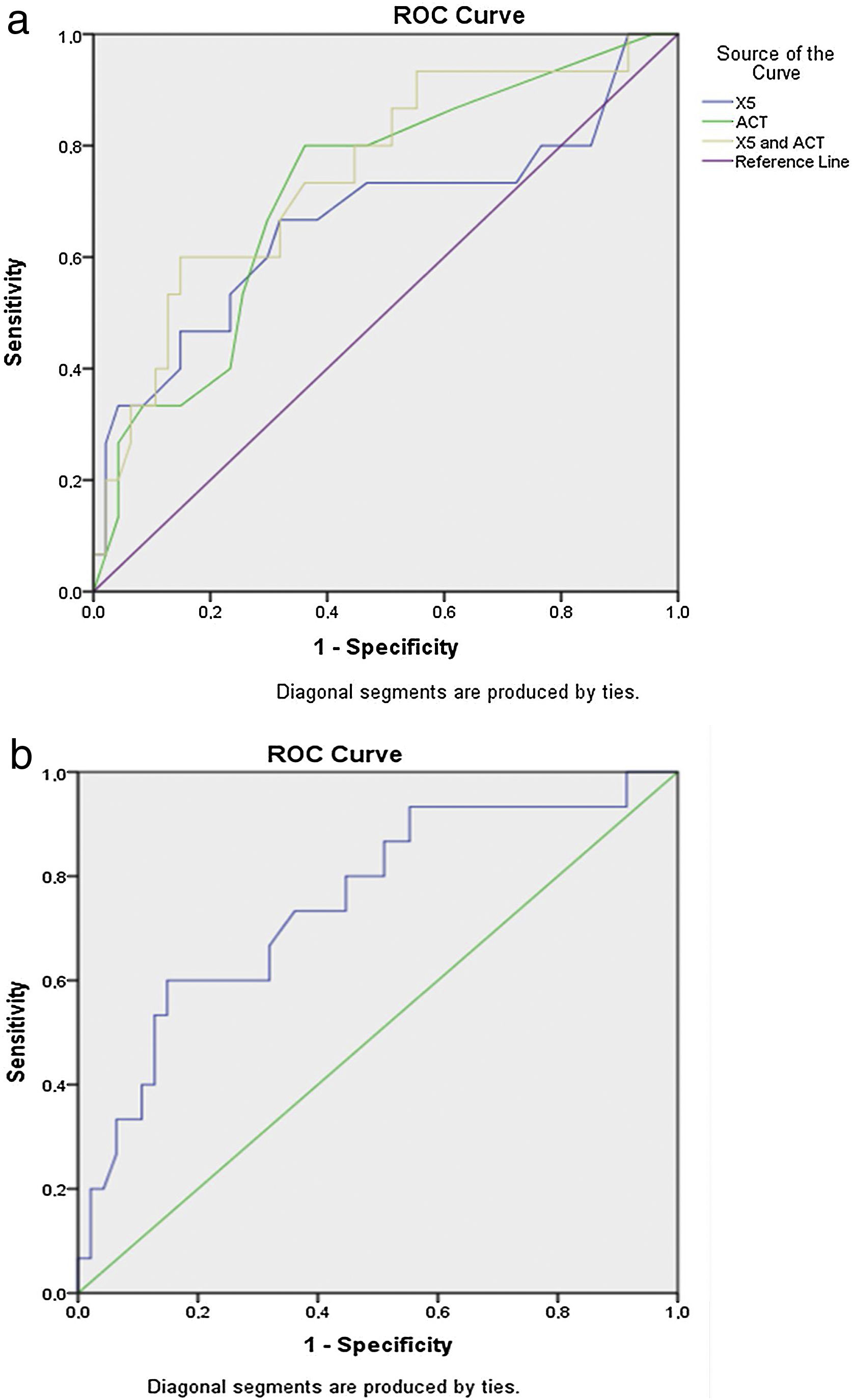
In total 64 consecutive patients with moderate asthma were evaluated, of whom 15 (23.4%) presented a PD20<800μg. Using the aforementioned cut points, the proportions of patients with SAD did not differ according to the presence of BHR (Supplementary Table S1). Using a logistic univariate regression model, significant predictors of the presence of BHR were reactance at 5Hz in IOS (X5) (p=0.035), the difference between resistance in 5Hz and 20Hz (R5-R20) (p=0.036) and ACT (p=0.013). The accuracy of the probabilistic models built for each parameter that was significant in the univariate analysis in predicting the presence of BHR expressed as AUC (95%CI) was 0.669 (0.488–0.849) for X5, 0.642 (0.460–0.823) for R5-R20 and 0.720 (0.572–0.867) for ACT (Fig. 1a).
Based on this, two multivariate probabilistic models were constructed. In the first multivariate model, the predictors included were those that were significant in univariate analysis (X5, R5-R20, ACT), providing an AUC of 0.729 (0.573–0.886), p=0.008. In the second multivariate model, the predictor R5-R20 was excluded, as the accuracy of the model built starting only from R5-R20 lost its significance (p=0.100) It was verified that the second model including X5 and ACT gained in accuracy and in significance with an AUC 0.750 (0.604–0.895), p=0.004 (Fig. 1b).
Based on the two significant predictors X5 and ACT, we constructed the following predictive equation for the detection of clinically significant BHR:
The addition to the model of markers of T2-inflammation such as FeNO and blood eosinophils (parameters that were not significant in the univariate logistic regression analysis), did not significantly improve the accuracy of the model (AUC=0.760 (0.585–0.936); p=0.022) compared to the one constructed with only the X5 and ACT parameters (AUC: 0.750 (0.604–0.895); p=0.004). A slight worsening of the p value was also observed, but always within the limits of statistical significance.
The methacholine bronchoprovocation test is a diagnostic test that requires a long time to run and to be performed in a protected hospital setting. As the presence of bronchial hyperresponsiveness is a marker of poor asthma control, and as SAD has gained increasing interest, mainly in association with gaining optimal asthma control,9 the equation could provide the probability to have BHR<800μg. The objective of this equation is to predict the presence of bronchial hyperreactivity by means of two simpler and safer diagnostic tests that can also be performed even outside the hospital environment: the ACT and the oscillometric reactance measurement which allows to derive the parameter X5. In this sense, the possibility can be configured in a patient who presents an ACT questionnaire in the control range of asthma but also presents an alteration of the function parameter of the small airways X5. The equation would allow the presence of residual bronchial hyperresponsiveness to be predicted more quickly and safely, thus ensuring therapeutic optimization. From a strictly pathophysiological point of view, this equation allows to highlight the presence of a link between small airways by means of the parameter X5, bronchial hyperresponsiveness and control of asthma.
In adult patients, IOS parameters were useful in assessing BHR but not in predicting this feature in patients already diagnosed with asthma.5 The same applies for the combination of FeNO and FEF25–75% or FEF50% which improved the diagnostic rate of mild asthma.7 Frequency dependence of resistance (R5-R20), FeNO and RV% predicted, have found to be reliable markers for prediction of mild to severe methacholine induced BHR but in a different population consisted of children aged 6-18 years with well-controlled asthma.6 Combinations of questionnaires and biomarkers have been previously used to define asthma control. For example, the combined use of FeNO and ACT has been shown to be a better predictor of GINA-defined asthma control than FeNO alone in a study in Vietnam.10
The present study is differentiated in that a questionnaire reflecting asthma control (ACT) and an IOS parameter (X5) representative of SAD, could predict the presence of residual BHR giving additional information on the overall asthma control and on the intent to reduce the anti-inflammatory treatment. This is further supported by a recently published study which revealed that adults with moderate-to-severe asthma with combined impairment of X5 and FEV1 had significantly worse asthma control.11
Our study has the potential limitation of the small number of participants; nevertheless, the proper characterization and the meticulous performance of different laboratory techniques for the evaluation of small airways disease provide reassurance for the quality of our results.
In conclusion, in this cross-sectional study of well characterized patients with asthma, we were able to show that the combination of a measure of asthma control (ACT) with an oscillometric measure of small airways disease (X5) may serve as predictors of the presence of bronchial hyperresponsiveness. Our results need to be validated in larger studies of well-characterized patients with asthma.
Conflict of interestAuthors declare no conflict of interest.
Dr Katsoulis has received honoraria for presentations and consultancy fees from AstraZeneca, Chiesi, GSK, Menarini, and Novartis.
Dr Kipourou has received honoraria for presentations from Astra Zeneca.
Dr Vitaliano Nicola Quaranta declares having no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript.
Prof Kostikas has received honoraria for presentations and consultancy fees from AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, ELPEN, GILEAD, GSK, Menarini, Novartis and Sanofi (paid to the University of Ioannina)
Prof Kostikas’ department has received funding and grants from AstraZeneca, Boehringer Ingelheim, Chiesi, Innovis, ELPEN, GSK, Menarini, Novartis and NuvoAir (paid to the University of Ioannina).