Available evidence suggests a familial basis for OSA. The aim of the present study was to assess the potential influences of parental OSA in predicting the diagnosis and severity of OSA in snoring children.
MethodsObservational study, we prospectively enrolled 84 children and their parents. A complete nocturnal polysomnography was performed. Children were categorized into 3 severity groups according to the apnea–hypopnea index (AHI<1h−1, AHI≥1h−1 to AHI<5h−1, and AHI≥5h−1). Adults were grouped according two criteria (AHI≥5h−1 and ≥10h−1).
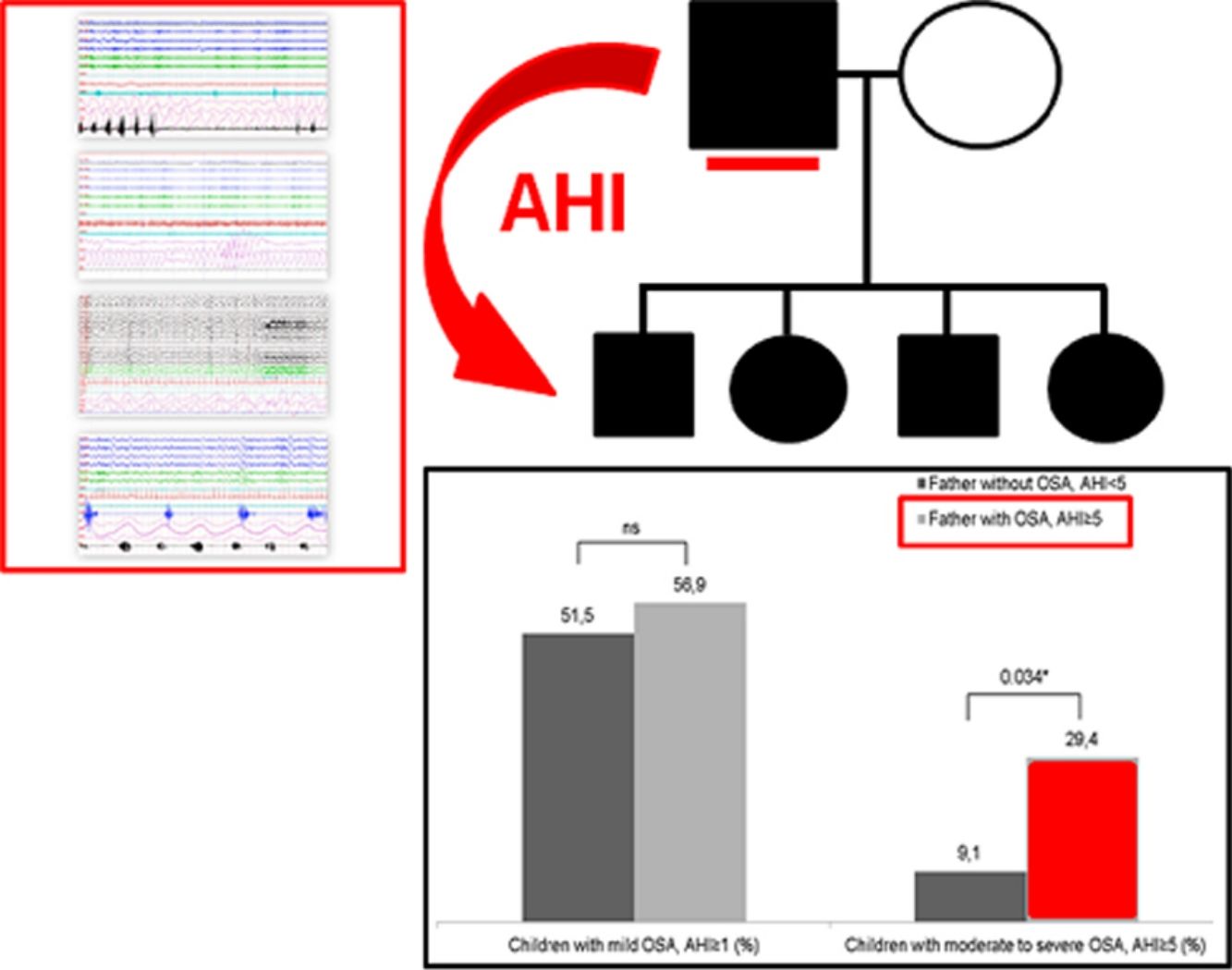
ResultsThere were no significant differences in age, gender, BMI and BMI z-score among groups. Among the children, 54.7% had an AHI≥1h−1 and 21.4% had an AHI≥5h−1. Overall, we observed that 60.7% of fathers and 23.8% of mothers of our population had OSA (AHI≥5h−1). The prevalence of fathers with OSA increases with the children's severity (83% in the group of children with moderate-severe OSA, p=0.035). The odds of having moderate-severe pediatric OSA (AHI≥5h−1) were more than 4 times higher among children with a father with AHI≥5h−1 (OR: 4.92, 95% CI: 1.27–19.06; p=0.021). There was no evidence of any maternal influence on OSA severity among the children studied.
ConclusionsOur findings suggest a high prevalence of OSA among the family members studied with an increased association of childhood OSA with paternal OSA. Prediction of OSA risk among children can be significantly improved by adding data on paternal OSA status.
La evidencia disponible sugiere una base familiar para la AOS. El objetivo del presente estudio fue evaluar las posibles influencias de la AOS de los padres para predecir el diagnóstico y la gravedad de la AOS en los niños que roncan.
MétodosEstudio observacional en el que incluimos prospectivamente a 84 niños y sus padres. Se realizó una polisomnografía nocturna completa. Los niños se clasificaron en 3 grupos de gravedad según el índice de apnea-hipopnea (IAH <1h−1, IAH ≥1h−1 a IAH <5h−1y IAH ≥5h−1). Los adultos se agruparon según dos criterios (IAH ≥5 h-1 y ≥10 h-1).
ResultadosNo había diferencias significativas en la edad, el sexo, el IMC y la puntuación z del IMC entre los grupos. Entre los niños, el 54,7% tenía un IAH ≥1h−1 y el 21,4% tenía un IAH ≥5h−1. En general, observamos que el 60,7% de los padres y el 23,8% de las madres de nuestra población tenían AOS (IAH ≥5h−1). La prevalencia de padres con AOS aumenta con la gravedad de la AOS en los niños (83% en el grupo de niños con AOS moderada-grave, p=0,035). La probabilidad de tener AOS pediátrica moderada-grave (IAH ≥5h−1) fue más de 4 veces mayor en los niños con un padre con IAH≥5h−1 (OR: 4,92, IC 95%: 1,27-19,06; p=0,021). No hubo evidencia de que hubiera alguna influencia materna en la gravedad de la AOS en los niños estudiados.
ConclusionesNuestros hallazgos sugieren una alta prevalencia de AOS entre los miembros de la familia estudiados con una mayor asociación de la AOS infantil con la AOS paterna. La predicción del riesgo de AOS entre los niños puede mejorarse significativamente al incluir información sobre el estado de la AOS paterna.
Obstructive sleep apnea (OSA) is a prevalent disorder that affects both adults and children.1,2 This disorder is characterized by episodic upper airway obstruction during sleep, resulting in recurrent episodes of intermittent hypoxia and repeated arousals. Clinically, OSA is characterized by snoring, daytime sleepiness and an impairment of quality of life and has been implicated as a risk factor for the development of cardiovascular and metabolic outcomes. In addition, a broad spectrum of adverse consequences in the development during childhood has been associated with OSA, including cognitive and behavioral problems, and a decrease in the quality of life.3–5
The prevalence and clinical relevance of OSA among children has increased over the past decade.6 Although adenotonsillar hypertrophy is the major contributor to the pathophysiology of OSA in the pediatric age, environmental and genetic factors potentially interact in the pathogenesis of pediatric OSA.7–9 Nevertheless, there are limited data regarding the identification of risk factors which may assist in the assessment of susceptible pediatric population.10 The identification of specific phenotypes including individual, familial and environmental variables could help in the establishment of diagnostic algorithms and early therapeutic strategies. Available evidence suggests a familial basis for OSA.11–16 However, several limitations are seen in previous studies that need to be considered, such as the inclusion of either adult or children subjects only or the use of questionnaires to diagnose sleep apnea.
The aim of the present study was to assess the potential influences of paternal and maternal presence of OSA in predicting the diagnosis and severity of OSA across snoring children referred to a sleep unit.
MethodsThis study was conducted from January 2014 and December 2016 as a longitudinal familial study. The target population were snoring children recruited at the Pediatric Pulmonology Unit and their parents.
A total of consecutive 153 children referred due to a snoring condition were selected. The exclusion criteria include individuals with a chronic disease (except for asthma or well-controlled rhinitis), genetic syndromes as well as those under medications potentially affecting sleep (hypnotics, relaxants, anxiolytics, antidepressants, opiates and antiepileptics), patients with missing or no biological paternity data, and history of tonsillo-adenoidectomy were excluded. Parents signed the informed consent, completed a demographic and medical questionnaire for their child and were invited to participate in the study. In adulthood, exclusion criteria, based on a medical questionnaire, were: presence of a cardiovascular chronic disease (heart failure, myocardial infarction, pulmonary embolism and arrhythmia), respiratory chronic disease, OSA in treatment or taking medications which would affect sleep or nocturnal respiration. A total of 84 families were included (children and their biological parents) and underwent screening polysomnography (PSG), most of the excluded families (n=69) were due to history of tonsillo-adenoidectomy (28%) and loss of parental involvement (72%). The study was evaluated and approved by the Ethics Committee of our institution and registered as IB 2136/13.
Anthropometric data were recorded for each participant: age, gender, comorbidities and clinical factors. Body-mass index (BMI) was calculated, and their values were adjusted by means of z-score.17
Sleep studyA complete polysomnography was performed (Grael, Compumedics, Abbotsford, Australia) at the multidisciplinary Sleep Unit of our hospital, following the American Academy of Sleep Medicine guidelines.18 Up to 6 encephalogram channels were studied, as well as chin and anterior tibial electromyogram, bilateral electro-oculogram, heart rate by ECG, airflow monitoring by nasal pressure transducer and oronasal thermistor, chest and abdominal wall movement by respiratory inductance plethysmography. Transcutaneous measurement of arterial oxygen saturation (SatO2) was performed by pulse oximetry. All sleep studies were analyzed by the same two experts, according to pediatric-age validated criteria. Apnea was defined as a decrease in nasal flow ≥90% in at least two respiratory cycles. Hypopnea was defined as a decrease ≥30% followed by an arousal in electroencephalogram or an oxygen desaturation greater than 3%. The apnea–hypopnea index (AHI) was calculated by adding the number of apneas and hypopneas, divided by the hours of sleep, and were considered obstructive apneas in the calculation of AHI.
In adults, apnea was defined as an absence or reduction ≥90% of the respiratory signal of >10s duration in the presence or absence of respiratory effort detected by the thoraco-abdominal bands. Hypopnea was defined as a reduction ≥30% of the amplitude of the respiratory signal of >10s of duration or a marked decrease in the thoraco-abdominal summation that is accompanied by a desaturation (≥3%) or a micro-awakening in the electroencephalogram, and were considered obstructive and central apneas in the calculation of AHI.
Children were categorized into 3 severity groups according to the apnea–hypopnea index (AHI<1h−1, AHI≥1 to AHI<5h−1, and AHI≥5h−1). Adults with OSA were grouped according two criteria of AHI in the mild OSA subgroup (AHI≥5h−1 and ≥10h−1),19,20 in order to detect familial associations in the earliest stage of the disease. Family history of OSA was identified if the parents reported that OSA had been diagnosed in at least 1 relative member of either families (other than the mother and father of the child), and no adjustment of family size was done. Sleep study was done in children and parents in the same lab on different days, separated for a period of time not exceeding fifteen days.
Statistical analysisA descriptive analysis was performed, calculating absolute and relative frequencies for qualitative variables. Mean and standard deviation or median and interquartile range were used for the quantitative parameters. Variable normality was assessed using the Kolmogorov–Smirnov's test. For mean comparison, a Student's t-test was used when appropriate. If not, U Mann–Whitney tests were applied. Chi-squared analyses were used to compare categorical variables. Correlations were assessed using the Spearman's rho test. Simple and multivariate regression models were used to assess the effects of paternal and maternal OSA on increasing childhood OSA severity. Odds ratio was calculated using a bivariate logistic regression and adjusted by age, gender and BMI z-score. A p-value below 0.05 was considered statistical significant. All data were processed and analyzed using SPSS v. 20.0 (IBM, NY, USA).
Taking an estimated incidence of OSA in children of 7%1 and adults of 35%,31 we need to analyze a minimum of 84 subjects, was calculated with an alpha error of 5% and statistical power (1−β) of 90%, with the enrollment ratio of 1:1.
ResultsA total of 84 children referred due to a snoring condition and their parents (father and mother) were finally included. Of them, 95% were Caucasian and 28.6% did not have adenotonsillar hypertrophy, grade IV of adenotonsillar hypertrophy was present in 2.4% of the individuals, and there were no significant differences in the presence of adenotonsillar hypertrophy across the OSA severity groups.
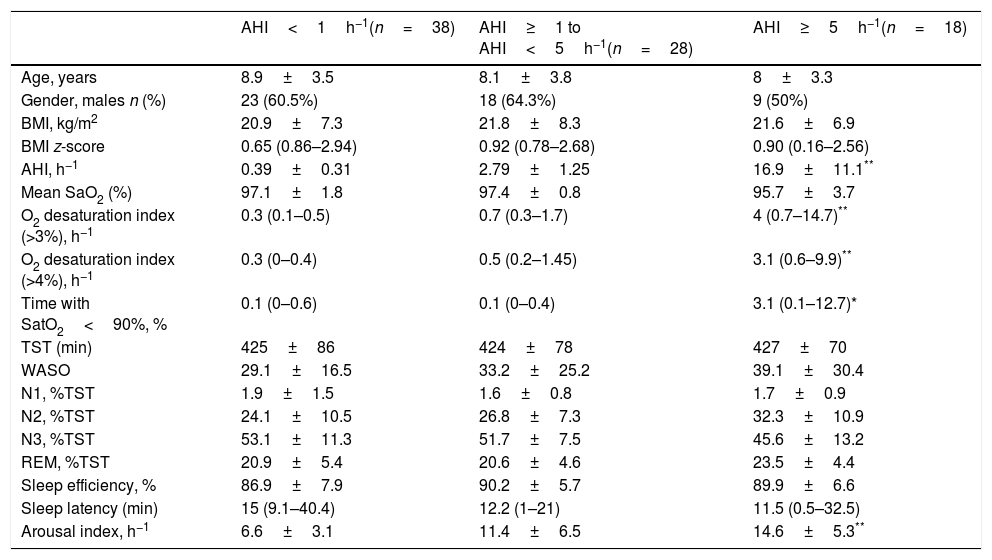
Table 1 illustrates the distributions of children characteristics according to OSA severity. There were no significant differences in age, gender, BMI and BMI z-score among groups. The sleep study yielded a 54.7% prevalence of pediatric OSA (AHI≥1h−1), and 21.4% of our children was classified as moderate-severe OSA (AHI≥5h−1). In these OSA groups, BMI z-score is higher than the group of non-OSA, but those differences do not reach statistical significance between the three groups.
Children characteristics according to OSA severity.
| AHI<1h−1(n=38) | AHI≥1 to AHI<5h−1(n=28) | AHI≥5h−1(n=18) | |
|---|---|---|---|
| Age, years | 8.9±3.5 | 8.1±3.8 | 8±3.3 |
| Gender, males n (%) | 23 (60.5%) | 18 (64.3%) | 9 (50%) |
| BMI, kg/m2 | 20.9±7.3 | 21.8±8.3 | 21.6±6.9 |
| BMI z-score | 0.65 (0.86–2.94) | 0.92 (0.78–2.68) | 0.90 (0.16–2.56) |
| AHI, h−1 | 0.39±0.31 | 2.79±1.25 | 16.9±11.1** |
| Mean SaO2 (%) | 97.1±1.8 | 97.4±0.8 | 95.7±3.7 |
| O2 desaturation index (>3%), h−1 | 0.3 (0.1–0.5) | 0.7 (0.3–1.7) | 4 (0.7–14.7)** |
| O2 desaturation index (>4%), h−1 | 0.3 (0–0.4) | 0.5 (0.2–1.45) | 3.1 (0.6–9.9)** |
| Time with SatO2<90%, % | 0.1 (0–0.6) | 0.1 (0–0.4) | 3.1 (0.1–12.7)* |
| TST (min) | 425±86 | 424±78 | 427±70 |
| WASO | 29.1±16.5 | 33.2±25.2 | 39.1±30.4 |
| N1, %TST | 1.9±1.5 | 1.6±0.8 | 1.7±0.9 |
| N2, %TST | 24.1±10.5 | 26.8±7.3 | 32.3±10.9 |
| N3, %TST | 53.1±11.3 | 51.7±7.5 | 45.6±13.2 |
| REM, %TST | 20.9±5.4 | 20.6±4.6 | 23.5±4.4 |
| Sleep efficiency, % | 86.9±7.9 | 90.2±5.7 | 89.9±6.6 |
| Sleep latency (min) | 15 (9.1–40.4) | 12.2 (1–21) | 11.5 (0.5–32.5) |
| Arousal index, h−1 | 6.6±3.1 | 11.4±6.5 | 14.6±5.3** |
BMI: body mass index, AHI: apnea-hipopnea index, TST: total sleep time. N1: sleep stage 1, N2 sleep stage2, N3: sleep stage 3, REM: rapid eye movement, WASO: wake after sleep onset. Data are presented as n (percentage) for categorical data or as mean±standard deviation or median (interquartile range) for continuous data.
60.7% and 54.7% of fathers had an AHI≥5 or ≥10h−1 respectively; 23.8 and 16.6% of mothers had an AHI≥5 or ≥10h−1 respectively.
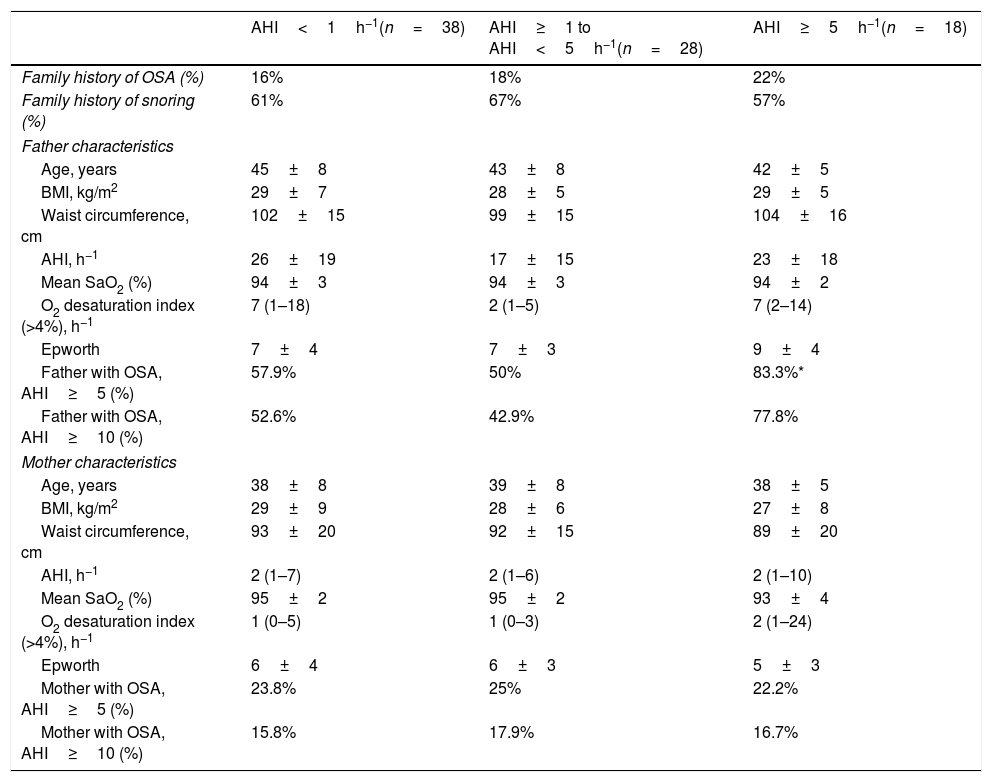
Table 2 provides the paternal and maternal status among the children studied. There were no significant differences in age, BMI, waist circumference and AHI among groups. When father assessments were made, the presence of mild OSA (AHI≥5h−1) was 57.9%, 50% and 83.3% respectively, among the 3 children groups (p=0.035). In comparison, there were no significant differences in maternal OSA distribution among the 3 children groups. Based on direct questions of medical questionnaire, there was no differences of familial history of snoring nor OSA diagnostic among groups.
Paternal and maternal status in snoring and pediatric OSA.
| AHI<1h−1(n=38) | AHI≥1 to AHI<5h−1(n=28) | AHI≥5h−1(n=18) | |
|---|---|---|---|
| Family history of OSA (%) | 16% | 18% | 22% |
| Family history of snoring (%) | 61% | 67% | 57% |
| Father characteristics | |||
| Age, years | 45±8 | 43±8 | 42±5 |
| BMI, kg/m2 | 29±7 | 28±5 | 29±5 |
| Waist circumference, cm | 102±15 | 99±15 | 104±16 |
| AHI, h−1 | 26±19 | 17±15 | 23±18 |
| Mean SaO2 (%) | 94±3 | 94±3 | 94±2 |
| O2 desaturation index (>4%), h−1 | 7 (1–18) | 2 (1–5) | 7 (2–14) |
| Epworth | 7±4 | 7±3 | 9±4 |
| Father with OSA, AHI≥5 (%) | 57.9% | 50% | 83.3%* |
| Father with OSA, AHI≥10 (%) | 52.6% | 42.9% | 77.8% |
| Mother characteristics | |||
| Age, years | 38±8 | 39±8 | 38±5 |
| BMI, kg/m2 | 29±9 | 28±6 | 27±8 |
| Waist circumference, cm | 93±20 | 92±15 | 89±20 |
| AHI, h−1 | 2 (1–7) | 2 (1–6) | 2 (1–10) |
| Mean SaO2 (%) | 95±2 | 95±2 | 93±4 |
| O2 desaturation index (>4%), h−1 | 1 (0–5) | 1 (0–3) | 2 (1–24) |
| Epworth | 6±4 | 6±3 | 5±3 |
| Mother with OSA, AHI≥5 (%) | 23.8% | 25% | 22.2% |
| Mother with OSA, AHI≥10 (%) | 15.8% | 17.9% | 16.7% |
BMI: body mass index, AHI: apnea–hypopnea index. Data are presented as n (percentage) for categorical data or as mean±standard deviation or median (interquartile range) for continuous data.
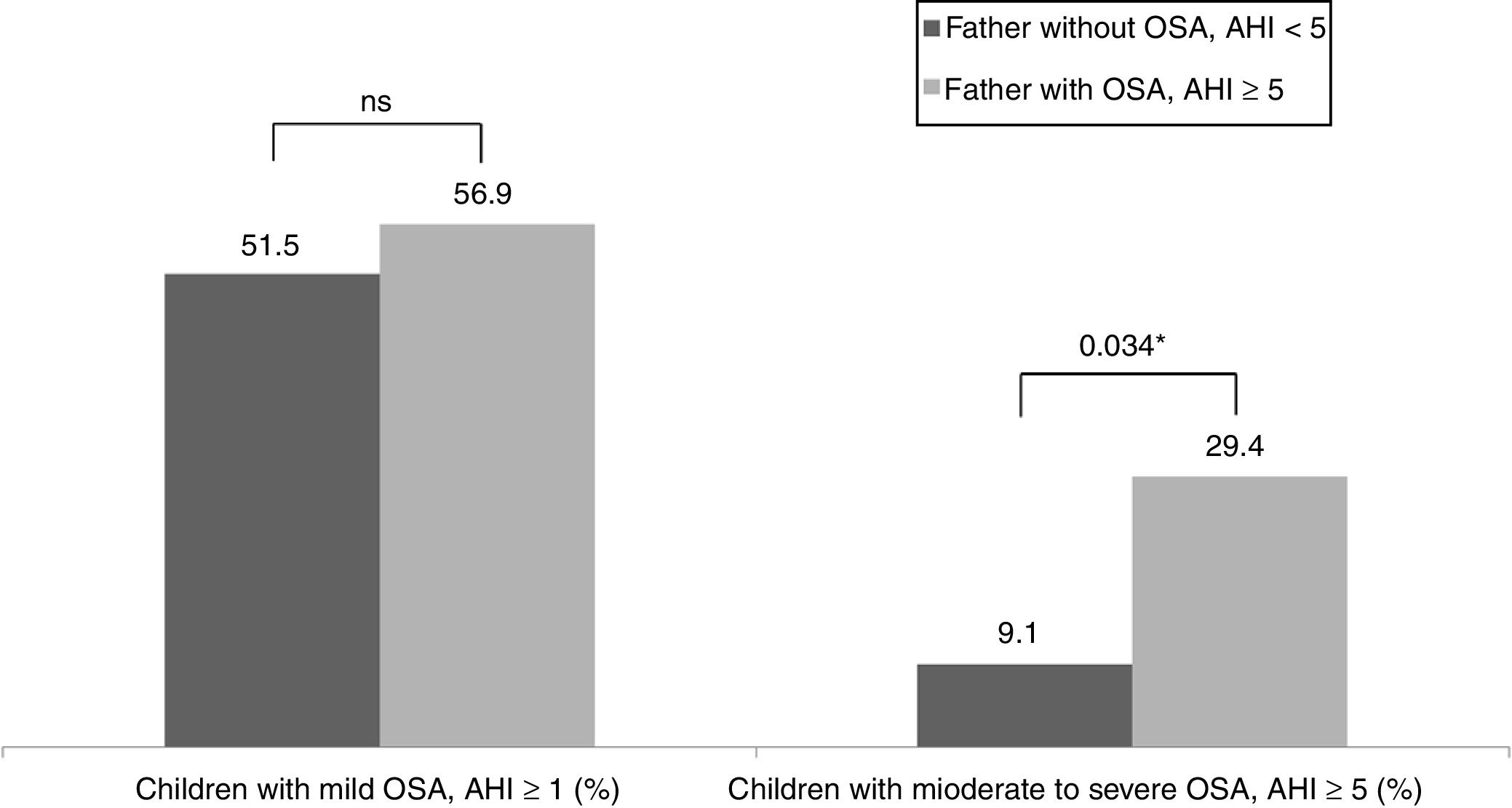
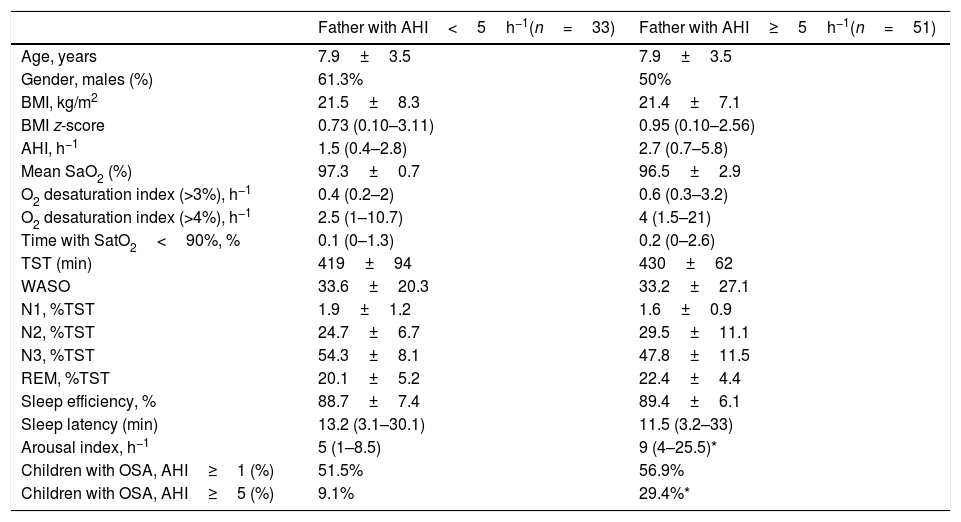
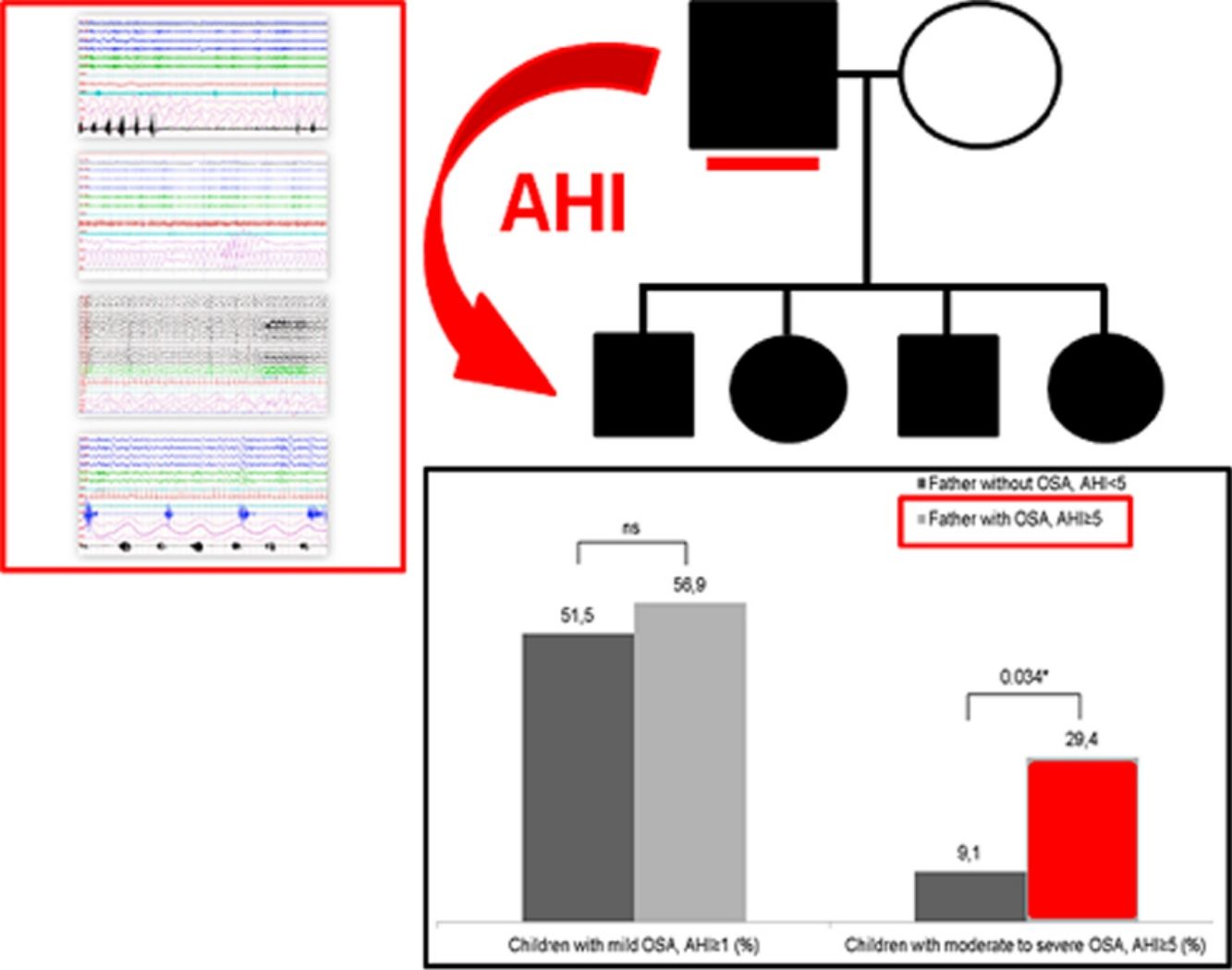
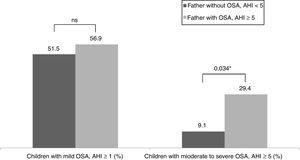
Table 3 shows the children characteristics based on the father's OSA status. There were significant differences in the arousal index between children with the father with OSA and children with the father without OSA. The percentage of children with a moderate-severe OSA was higher in the group with the father with OSA (p=0.034) (Fig. 1).
Children characteristics based on the OSA father status.
| Father with AHI<5h−1(n=33) | Father with AHI≥5h−1(n=51) | |
|---|---|---|
| Age, years | 7.9±3.5 | 7.9±3.5 |
| Gender, males (%) | 61.3% | 50% |
| BMI, kg/m2 | 21.5±8.3 | 21.4±7.1 |
| BMI z-score | 0.73 (0.10–3.11) | 0.95 (0.10–2.56) |
| AHI, h−1 | 1.5 (0.4–2.8) | 2.7 (0.7–5.8) |
| Mean SaO2 (%) | 97.3±0.7 | 96.5±2.9 |
| O2 desaturation index (>3%), h−1 | 0.4 (0.2–2) | 0.6 (0.3–3.2) |
| O2 desaturation index (>4%), h−1 | 2.5 (1–10.7) | 4 (1.5–21) |
| Time with SatO2<90%, % | 0.1 (0–1.3) | 0.2 (0–2.6) |
| TST (min) | 419±94 | 430±62 |
| WASO | 33.6±20.3 | 33.2±27.1 |
| N1, %TST | 1.9±1.2 | 1.6±0.9 |
| N2, %TST | 24.7±6.7 | 29.5±11.1 |
| N3, %TST | 54.3±8.1 | 47.8±11.5 |
| REM, %TST | 20.1±5.2 | 22.4±4.4 |
| Sleep efficiency, % | 88.7±7.4 | 89.4±6.1 |
| Sleep latency (min) | 13.2 (3.1–30.1) | 11.5 (3.2–33) |
| Arousal index, h−1 | 5 (1–8.5) | 9 (4–25.5)* |
| Children with OSA, AHI≥1 (%) | 51.5% | 56.9% |
| Children with OSA, AHI≥5 (%) | 9.1% | 29.4%* |
BMI: body mass index, AHI: apnea-hipopnea index, TST: total sleep time. N1: sleep stage 1, N2 sleep stage2, N3: sleep stage 3, REM: rapid eye movement, WASO: wake after sleep onset. Data are presented as n (percentage) for categorical data or as mean±standard deviation or median (interquartile range) for continuous data.
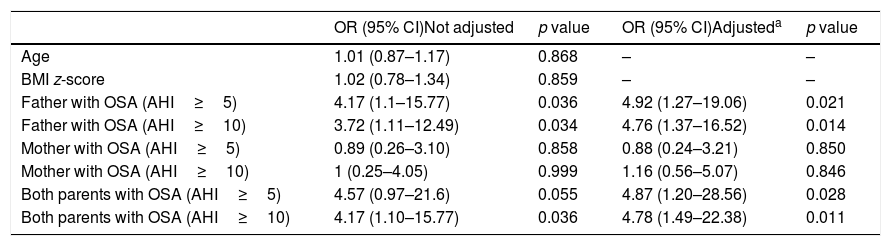
Overall, the odds of having moderate-severe OSA was more than 4 times higher among children with the father with OSA (Table 4). There was no evidence of any statistical interaction between maternal and paternal OSA and its influence on OSA severity among the children studied.
Risk of OSA in snoring children according parental OSA severity.
| OR (95% CI)Not adjusted | p value | OR (95% CI)Adjusteda | p value | |
|---|---|---|---|---|
| Age | 1.01 (0.87–1.17) | 0.868 | – | – |
| BMI z-score | 1.02 (0.78–1.34) | 0.859 | – | – |
| Father with OSA (AHI≥5) | 4.17 (1.1–15.77) | 0.036 | 4.92 (1.27–19.06) | 0.021 |
| Father with OSA (AHI≥10) | 3.72 (1.11–12.49) | 0.034 | 4.76 (1.37–16.52) | 0.014 |
| Mother with OSA (AHI≥5) | 0.89 (0.26–3.10) | 0.858 | 0.88 (0.24–3.21) | 0.850 |
| Mother with OSA (AHI≥10) | 1 (0.25–4.05) | 0.999 | 1.16 (0.56–5.07) | 0.846 |
| Both parents with OSA (AHI≥5) | 4.57 (0.97–21.6) | 0.055 | 4.87 (1.20–28.56) | 0.028 |
| Both parents with OSA (AHI≥10) | 4.17 (1.10–15.77) | 0.036 | 4.78 (1.49–22.38) | 0.011 |
OR: Odds ratio, BMI: body mass index, AHI: apnea–hypopnea index.
With the use of PSG, the focus of this study was to describe the distribution of familial OSA from a clinical sample of snoring children referred for diagnostic assessment of OSA. We considered the potential influences of paternal and maternal presence of OSA in predicting the diagnosis and severity of OSA in their children.
We also observed a wide spectrum of OSA, ranging from a few episodes of apnea per night to severe OSA with AHI >30h−1. Altogether, these findings are in line with previous reports indicating weak concordance between parents-reported symptoms of OSA and PSG variables21,22 and highlight the importance to identify screening strategies for childhood OSA.
Although a factor of heritability is involved in the pathogenesis of OSA, the effect of family on childhood OSA is poorly understood.23,24 Of interest was our finding of parental gender differences. We found that high AHI values in fathers are more prevalent in the group of children with severe OSA. By contrast, we found no significant associations of OSA status between mothers and their children. Previous studies suggested that a multiple pattern of sleep-disordered breathing was more common among female family members.12,25 The prevalence of OSA is influenced by gender and age.26,27 The age is important due to a greater probability of developing the predisposing disease over time. With age, the risk in men increases modestly, whereas that risk in women increases markedly.28,29 Changes in sex hormones have been pointed out as a cause of these differences.30 Since most mothers of our population were younger than 40 years the absence of an association may be the result of our analysis to women who did not have high risk to be affected with OSA.
Based on the 3 AHI categories in children, the percentage of mothers with OSA (AHI≥5h−1) was 23.8%, 25% and 22.2%, respectively. In comparison, the distribution of fathers with OSA (AHI≥5h−1) was 57.9%, 50% and 83.3%, respectively. Population studies demonstrate these differences between men and women according to the age subgroup and BMI.31 Thus, we found that, among fathers with snoring children, the prevalence of OSA increased in the severe OSA children category. Additionally, a varying influence of maternal and paternal OSA status on the offspring has been observed. Fathers rather than mothers have been shown to be a strong predictor of the OSA risk of their children. Overall, these findings suggest that there may potentially be separate physiological pathways through which the risk is conferred from each parent to the child.
The association between pediatric OSA and parental OSA seems to be an interplay between genetic and environmental influences.5,32,33 To understand the large variation in OSA phenotypes, a family history has been proposed as a tool to predict OSA risk. In general, studies focusing on familial predisposition of OSA use questionnaires or revise the medical records of the family members.13,34 The degree to which questionnaires may be used for identification of sleep apnea and family history is controversial. In our study, the absence of an association of family history may be the result of our restricting the analysis to individuals who did not have OSA at their initial examination.
With the use of PSG we detected that 60.7% of fathers and 23.8% of mothers of our population had OSA, hence suggesting that the data obtained by means of questionnaires could introduce an information bias from the parents. We also found that a snoring child with a father with OSA has a 4-time higher risk of having a moderate-severe OSA than a children with no affected father. Although, suggest that parents of snoring children should undergo a PSG to estimate the risk of their child having OSA is time consuming and not cost-effective. However, the presence of OSA parents should be taken into account to make a better indication of sleep studies in the snoring child.
This study identifies paternal AHI as a more prevalent condition in severe pediatric OSA. Indeed, the arousal index was higher in the children with an OSA father than children with a non-OSA father. Thus, it is possible that snoring children who experienced sleep disruption, and with an OSA father, may represent a phenotype with an increased risk for develop OSA or may therefore simply reflect more sleep fragmentation in children with OSA.
Family predisposition is considered as a combination of both an inherited and an environmental component between the family members.11,13,33,35 In the current study and in agreement with previous pediatric studies, age or gender were not associated with OSA.7,21 In addition, measures of adiposity were not associated with OSA, suggesting that other factors than obesity influence OSA susceptibility. Future research is needed to address which familial factors as a body composition, fat distribution and other measures beyond BMI, need to be considered as putative triggers for pediatric sleep apnea and different pediatric OSA phenotypes must be considered.
There are several limitations in our study. First, the adjustment for family size was not used in this study. Second, the fact that the study population included a small sample size of clinical snoring children with suspected OSA referred to a sleep unit could hamper the general applicability of the results and could imply a selection bias. However, the clinical relevance of our findings seems to be important given the high OSA prevalence found among the family members included.
We found that paternal OSA was associated with an increased risk of pediatric OSA severity. The results of this study found an association between pediatric and paternal OSA and suggest a factor of heritability for this syndrome. In the pediatric population, the role of genetic factors is unclear and many other variables could contribute to the accumulation of risk. In our study, the prediction for OSA in childhood is improved by obtaining a parental objective assessment of sleep. Third, we did not evaluate children who did not have biological parents and hence the results cannot be utilized for families involving grandparent or other family members as the primary caretaker of the child, and not evaluated the craniofacial features to support a genetic influence in the study findings. Fourth, based on the high prevalence of snorers in the family (including parents) a cut-off of 15h−1 and 30h−1 of AHI may be a more useful definition for severity groups in adults with OSA.
ConclusionsIn summary, based on our data, the prediction of OSA risk among snoring children can be significantly improved by adding data on paternal OSA status. The high prevalence of familial OSA makes it important to characterize OSA phenotypes in childhood that may lead to a better diagnosis and treatment of this condition.
AuthorshipAll authors must have contributed substantially to the conception and design of the study, the acquisition of data, or the analysis and interpretation of the data. Drafted or provided critical revision of the article. Provided final approval of the version submitted for publication.
Author contributions: AB, DMG, AA and JAPZ conceived and designed the study. AB, DMG, JP, PG, CR, NT and JAPZ supervised the data collection and managed the data, including quality control. PS, MP, JMB and AB provided statistical advice on study design and analyzed the data, AB and AA chaired the data oversight committee. AB and DMG drafted the manuscript, and all authors contributed substantially to its revision. AB and AA takes responsibility for the paper as a whole.
FundingThis work was supported by: Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spain [Grant: FIS PI1302120].
Conflict of interestThe authors declare no conflict of interest.