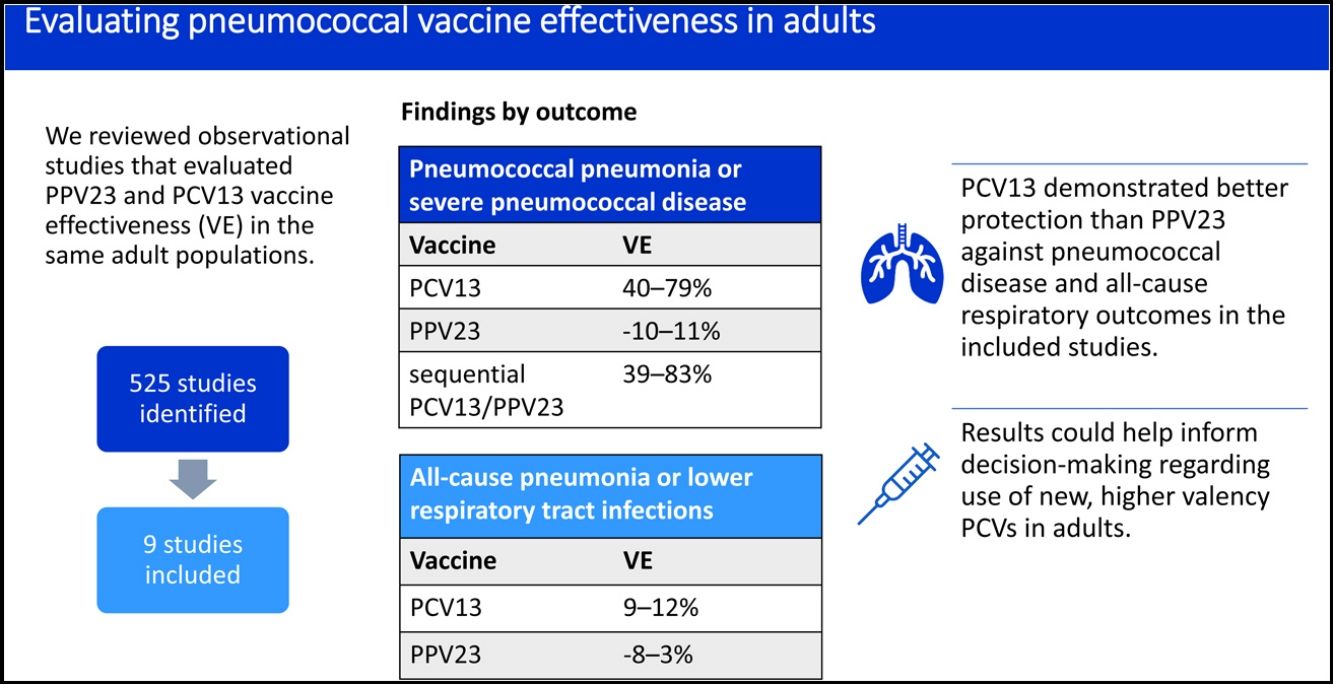
Fifteen and 20-valent pneumococcal conjugate vaccines (PCV15; PCV20) were recently licensed to prevent pneumococcal disease in adults. In the absence of efficacy or effectiveness data for these new vaccines, studies comparing 23-valent pneumococcal polysaccharide vaccine (PPV23) and PCV13 might help inform decision-making on how to best implement expanded-valency PCVs. Comparing PPV23 and PCV13 is problematic, as no head-to-head clinical trials evaluated efficacy. Comparing effectiveness results across observational studies that vary by population, design, and outcomes is difficult. To address these limitations, we undertook a narrative review of studies that assessed PPV23 and PCV13 vaccine effectiveness (VE) in the same adult populations.
MethodsWe conducted a literature search in PubMed and Google Scholar and screened 525 studies using a standardized evaluation framework.
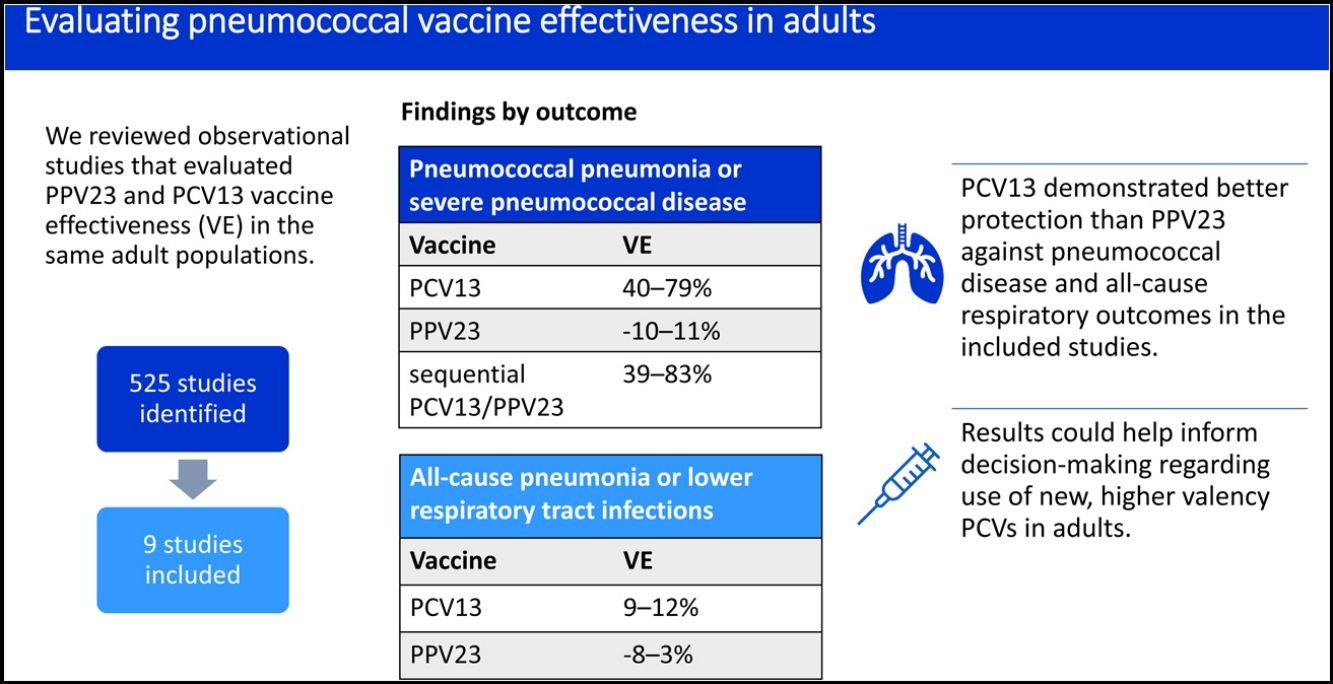
ResultsNine studies met inclusion criteria, all from high-income countries. None evaluated invasive pneumococcal disease (IPD) alone. VE against vaccine-type pneumococcal pneumonia ranged from 2 to 6% for PPV23 and 41 to 71% for PCV13. VE against pneumococcal pneumonia or severe pneumococcal disease (IPD or pneumococcal pneumonia) ranged from −10 to 11% for PPV23, 40 to 79% for PCV13, and 39 to 83% for sequential PCV13/PPV23. VE against all-cause pneumonia or lower respiratory tract infection ranged from −8 to 3% for PPV23 and 9 to 12% for PCV13.
ConclusionsOverall, PCV13 demonstrated better protection than PPV23 against pneumococcal disease and all-cause respiratory outcomes in the included studies. Where evaluated, sequential PCV13/PPV23 vaccination showed little benefit over PCV13 alone. Results support the use of PCVs to protect against pneumococcal disease and respiratory infections in adults.
Streptococcus pneumoniae (the pneumococcus) is a significant cause of disease worldwide that primarily affects young children and older adults.1 Pneumococcal infections include invasive pneumococcal disease (IPD) such as bacteremia and meningitis as well as non-invasive diseases including non-bacteremic pneumonia. Two classes of pneumococcal vaccines are licensed for use in adults. The 23-valent pneumococcal polysaccharide vaccine (PPV23) was first licensed in 1983. The 13-valent pneumococcal conjugate vaccine (PCV13), which contains capsular polysaccharides conjugated to a protein carrier, was licensed for adults in 2011. Higher valency pneumococcal conjugate vaccines (PCV15 and PCV20) were approved in the United States and Europe during 2021–2022 but have not yet been widely used.2,3
Pneumococcal vaccines are most commonly used for older adults or those with certain underlying conditions in high- or middle-income countries.4 Recommendations for adult pneumococcal vaccination vary by country, in terms of age group, risk categories, vaccine formulation, schedule (single vaccination or sequential vaccination with PPV23 followed by PCV), and reimbursement policies. Evidence is needed to inform clinical and public health decision making, as using multiple vaccines targeting the same pathogen increases costs and complicates recommendations.5 For PCV15 and PCV20, no pre-licensure efficacy trials against disease outcomes were conducted, and post-licensure effectiveness data are not yet available. Therefore, data on PPV23 and PCV13 might help inform decision-making. No head-to-head randomized controlled trials directly compared the clinical efficacy of PPV23 and PCV13. Several observational studies have evaluated the real-world effectiveness of PPV23 and PCV13, with results summarized in systematic reviews.6,7
Comparing vaccine effectiveness (VE) across observational studies is difficult due to differences in study design, outcomes, and duration of follow-up. Disease epidemiology including serotype distribution can differ by population, in part due to varying levels of indirect protection provided by pediatric PCV programs. Observational studies evaluating the effectiveness of PPV23 and PCV13 within the same adult population may offer key opportunities for direct comparison of these vaccines. Such studies reduce some of the inherent variability of observational studies with differing methodologies, pneumococcal vaccine utilization, and disease epidemiology across distinct populations. In this narrative literature review, we assessed observational studies that investigated the effectiveness of PPV23 and PCV13 within the same population using consistent methodologies for a range of clinical outcomes, including etiologically-confirmed pneumococcal diseases and clinically-defined respiratory outcomes such as all-cause pneumonia and lower respiratory tract infection (LRTI).
MethodsStudy IdentificationWe identified scientific publications published between January 2015 and December 2021 in which PPV23 and PCV13 effectiveness against pneumococcal disease or lower respiratory tract infection outcomes were investigated in the same adult population, either within a single study or in separate studies conducted by the same investigators using consistent methods. No studies assessing PCV13 effectiveness in adults were identified prior to 2015. A search strategy and evaluation framework facilitated identification of relevant studies and established criteria for inclusion. We conducted literature searches in PubMed and Google Scholar in December 2021 using a combination of the following search terms:’13-valent pneumococcal vaccine’, ‘PCV13’, 23-valent pneumococcal polysaccharide vaccine’, ‘PPV23’, ‘PPSV23’, ‘adult’, ‘vaccine effectiveness’, ‘pneumococcal disease’, ‘pneumonia’, ‘observational’. The terms ‘cost-effectiveness’, ‘PCV15’, and ‘immunogenicity’ were excluded from Google Scholar searches to reduce out-of-scope results. Additionally, we included relevant studies identified by co-authors and examined published systematic reviews on adult pneumococcal vaccination for eligible studies that may have been missed.
Study AssessmentWe used a standardized evaluation framework to assess studies. First, titles were reviewed for relevance, followed by abstract review. Studies not in scope (such as studies examining the indirect effects of pediatric PCV programs on adult disease) were excluded. We did not assess mortality outcomes as part of this review. For remaining studies, the full text was examined and information on design, study population and vaccination history, outcomes, adjustments for confounding, and results were extracted. Title and abstract review and data extraction were conducted by a single evaluator (EMD).
Next, we applied the following exclusion criteria intended to rule out studies with high risk of bias: lack of adjusted analyses or other strategy to address potential confounding, insufficient methods description, or low pneumococcal vaccination rates in the study population. The World Health Organization recommends a vaccination coverage level between 20% and 80% for case–control studies measuring the impact of pediatric PCV vaccination.8 As coverage of PCV13 among adults is below 20% in many countries, we used a cutoff of 5%, taking into consideration matching or other strategies used by investigators to ensure that vaccinated participants were generally similar to unvaccinated participants. Lastly, the remaining studies were assessed using the risk of bias in non-randomized studies of interventions (ROBINS-I) tool.9 All authors reviewed and agreed with the selection of included studies.
AnalysisVE was either defined and reported in the original study or calculated using standard epidemiologic methods as described in Supplementary Table 1. All VE estimates were derived from adjusted or matched analyses to reduce potential confounding (unless otherwise noted) and reported with 95% confidence intervals (CI). VE estimates are presented individually for each study by vaccine type (PCV13, PPV23, or sequential PCV13/PPV23), and categorized by outcome (e.g., vaccine-type disease, pneumococcal disease, all-cause pneumonia or LRTI, COVID-19). For studies that allowed for calculation of incidence rate reduction (IRR), these were calculated as the incidence among unvaccinated participants (annual incidence per 100,000 persons or incidence per 100,000 person-years)×VE, as previously described.10
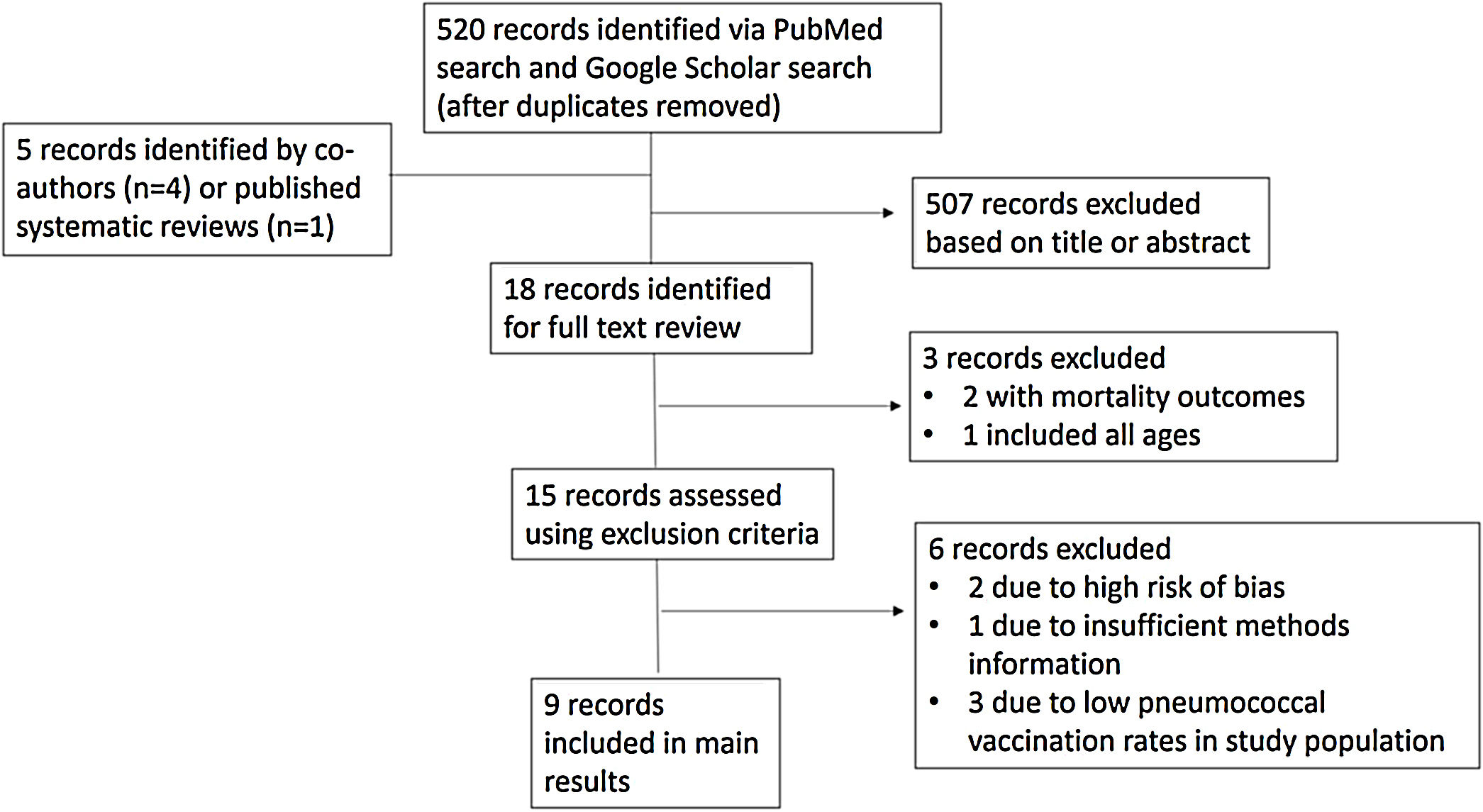
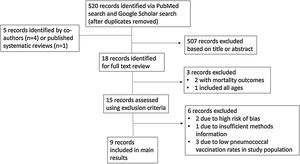
ResultsSearch Results and Study InclusionA total of 525 records were identified by our search; 507 were excluded following review of title and abstract (Fig. 1). Of the 18 records for which full text was reviewed, three were excluded as out of scope. The remaining 15 studies were evaluated according to the exclusion criteria. Six studies were excluded following full-text review, as described in Fig. 1 and Supplementary Table 2. Nine observational studies that investigated the effectiveness of PCV13 and PPV23 in the same or similar adult populations were included, with study characteristics summarized in Table 1. These nine studies were conducted in the United States, South Korea, and Germany, all high-income countries with well-established infant PCV immunization programs. Three studies were case–control studies using a test-negative design, whereas the other six were retrospective cohort studies that used administrative data. For five included studies, PCV13 and PPV23 effectiveness were examined contemporaneously, with results published in a single manuscript. Two pairs of studies, in which the same investigator team evaluated VE in the same target population using similar methods, with results for PCV13 and PPV23 published separately, were also included. Included studies adjusted for important confounders including age and underlying conditions. Additional study information including case definitions, outcome measurements, length of follow up, and results is provided in Supplementary Table 1. All included studies were categorized as having moderate risk of bias according to ROBINS-I.
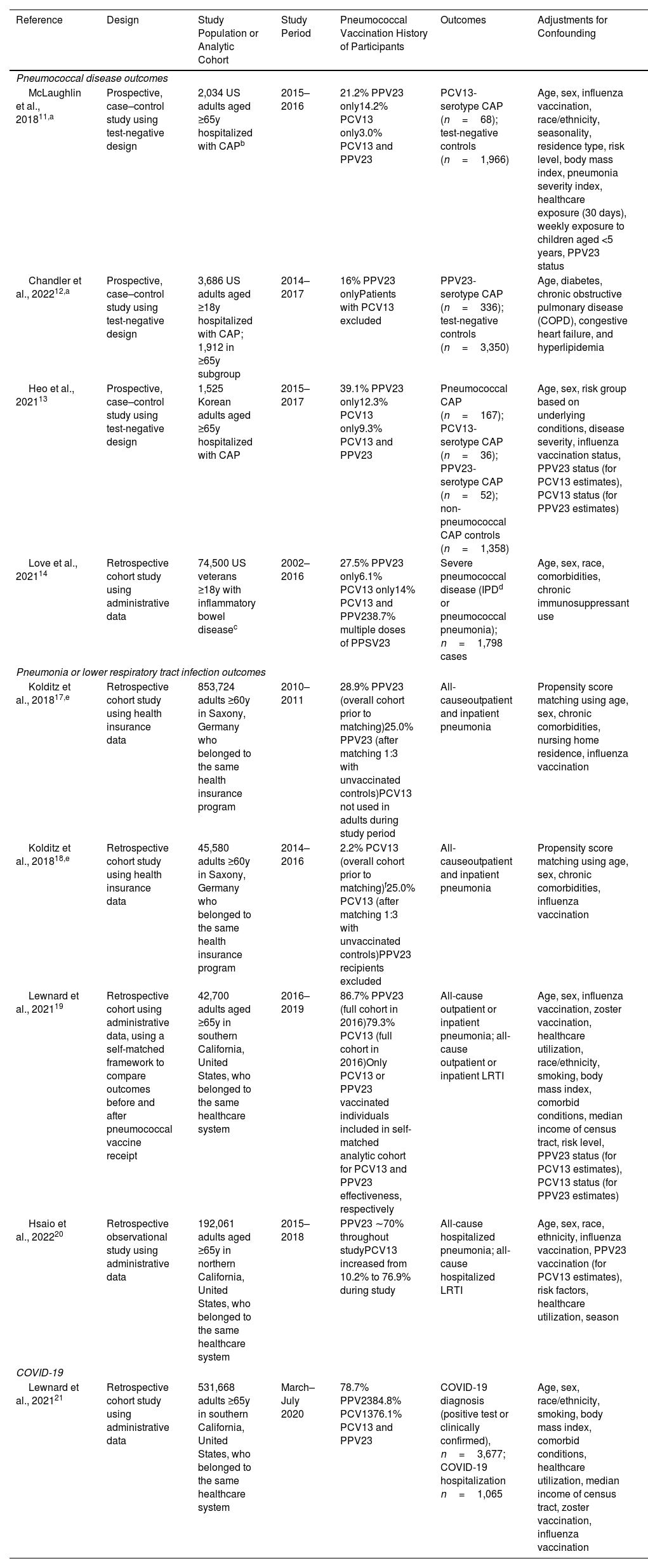
Characteristics of Included Observational Studies on Vaccine Effectiveness of PCV13 and PPV23 in the Same or Similar Populations.
| Reference | Design | Study Population or Analytic Cohort | Study Period | Pneumococcal Vaccination History of Participants | Outcomes | Adjustments for Confounding |
|---|---|---|---|---|---|---|
| Pneumococcal disease outcomes | ||||||
| McLaughlin et al., 201811,a | Prospective, case–control study using test-negative design | 2,034 US adults aged ≥65y hospitalized with CAPb | 2015–2016 | 21.2% PPV23 only14.2% PCV13 only3.0% PCV13 and PPV23 | PCV13-serotype CAP (n=68); test-negative controls (n=1,966) | Age, sex, influenza vaccination, race/ethnicity, seasonality, residence type, risk level, body mass index, pneumonia severity index, healthcare exposure (30 days), weekly exposure to children aged <5 years, PPV23 status |
| Chandler et al., 202212,a | Prospective, case–control study using test-negative design | 3,686 US adults aged ≥18y hospitalized with CAP; 1,912 in ≥65y subgroup | 2014–2017 | 16% PPV23 onlyPatients with PCV13 excluded | PPV23-serotype CAP (n=336); test-negative controls (n=3,350) | Age, diabetes, chronic obstructive pulmonary disease (COPD), congestive heart failure, and hyperlipidemia |
| Heo et al., 202113 | Prospective, case–control study using test-negative design | 1,525 Korean adults aged ≥65y hospitalized with CAP | 2015–2017 | 39.1% PPV23 only12.3% PCV13 only9.3% PCV13 and PPV23 | Pneumococcal CAP (n=167); PCV13-serotype CAP (n=36); PPV23-serotype CAP (n=52); non-pneumococcal CAP controls (n=1,358) | Age, sex, risk group based on underlying conditions, disease severity, influenza vaccination status, PPV23 status (for PCV13 estimates), PCV13 status (for PPV23 estimates) |
| Love et al., 202114 | Retrospective cohort study using administrative data | 74,500 US veterans ≥18y with inflammatory bowel diseasec | 2002–2016 | 27.5% PPV23 only6.1% PCV13 only14% PCV13 and PPV238.7% multiple doses of PPSV23 | Severe pneumococcal disease (IPDd or pneumococcal pneumonia); n=1,798 cases | Age, sex, race, comorbidities, chronic immunosuppressant use |
| Pneumonia or lower respiratory tract infection outcomes | ||||||
| Kolditz et al., 201817,e | Retrospective cohort study using health insurance data | 853,724 adults ≥60y in Saxony, Germany who belonged to the same health insurance program | 2010–2011 | 28.9% PPV23 (overall cohort prior to matching)25.0% PPV23 (after matching 1:3 with unvaccinated controls)PCV13 not used in adults during study period | All-causeoutpatient and inpatient pneumonia | Propensity score matching using age, sex, chronic comorbidities, nursing home residence, influenza vaccination |
| Kolditz et al., 201818,e | Retrospective cohort study using health insurance data | 45,580 adults ≥60y in Saxony, Germany who belonged to the same health insurance program | 2014–2016 | 2.2% PCV13 (overall cohort prior to matching)f25.0% PCV13 (after matching 1:3 with unvaccinated controls)PPV23 recipients excluded | All-causeoutpatient and inpatient pneumonia | Propensity score matching using age, sex, chronic comorbidities, influenza vaccination |
| Lewnard et al., 202119 | Retrospective cohort using administrative data, using a self-matched framework to compare outcomes before and after pneumococcal vaccine receipt | 42,700 adults aged ≥65y in southern California, United States, who belonged to the same healthcare system | 2016–2019 | 86.7% PPV23 (full cohort in 2016)79.3% PCV13 (full cohort in 2016)Only PCV13 or PPV23 vaccinated individuals included in self-matched analytic cohort for PCV13 and PPV23 effectiveness, respectively | All-cause outpatient or inpatient pneumonia; all-cause outpatient or inpatient LRTI | Age, sex, influenza vaccination, zoster vaccination, healthcare utilization, race/ethnicity, smoking, body mass index, comorbid conditions, median income of census tract, risk level, PPV23 status (for PCV13 estimates), PCV13 status (for PPV23 estimates) |
| Hsaio et al., 202220 | Retrospective observational study using administrative data | 192,061 adults aged ≥65y in northern California, United States, who belonged to the same healthcare system | 2015–2018 | PPV23 ∼70% throughout studyPCV13 increased from 10.2% to 76.9% during study | All-cause hospitalized pneumonia; all-cause hospitalized LRTI | Age, sex, race, ethnicity, influenza vaccination, PPV23 vaccination (for PCV13 estimates), risk factors, healthcare utilization, season |
| COVID-19 | ||||||
| Lewnard et al., 202121 | Retrospective cohort study using administrative data | 531,668 adults ≥65y in southern California, United States, who belonged to the same healthcare system | March–July 2020 | 78.7% PPV2384.8% PCV1376.1% PCV13 and PPV23 | COVID-19 diagnosis (positive test or clinically confirmed), n=3,677; COVID-19 hospitalization n=1,065 | Age, sex, race/ethnicity, smoking, body mass index, comorbid conditions, healthcare utilization, median income of census tract, zoster vaccination, influenza vaccination |
Paired studies conducted in the same setting (Louisville, Kentucky, United States) by the same investigator team; time frames of these two studies overlapped and consistent laboratory methods were used for case detection, with some minor differences in study design.
In the United States, adults with inflammatory bowel disease treated with immunosuppressing medications are recommended for pneumococcal vaccination at age ≥19 years due their immunocompromised status.40 Revaccination with PPV23 is recommended for adults with certain underlying conditions if the first dose was given before age 65 (https://www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf).
Paired studies conducted on the same database by the same investigator team using consistent methods; time frames of these two studies differed.
Although PCV13 vaccination coverage in the overall pupation was <5%, a matching strategy based on propensity scores resulted in an analytical cohort of which 25% of participants were PCV13-vaccinated and were broadly similar to unvaccinated controls with respect to known pneumococcal disease risk factors.
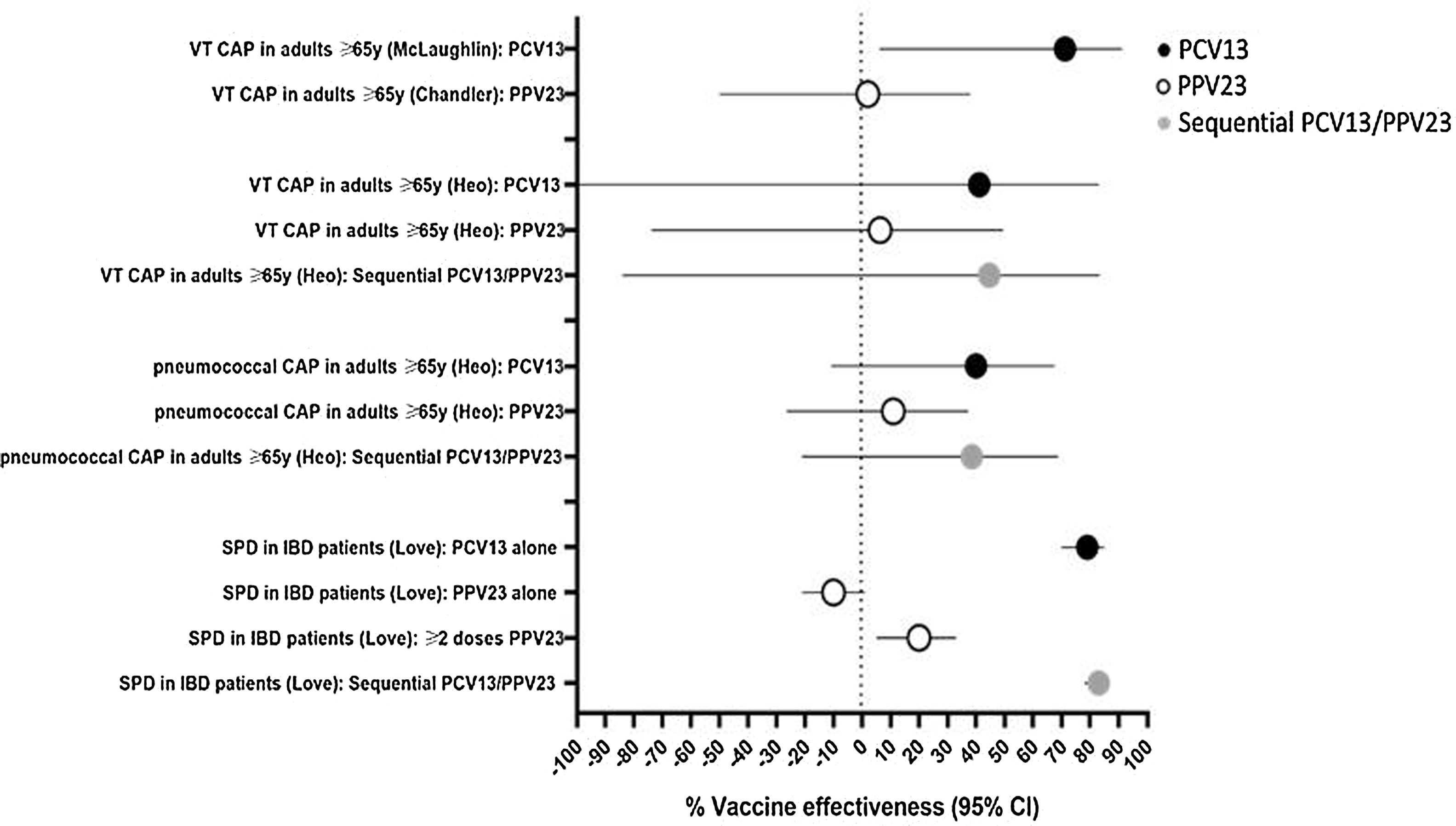
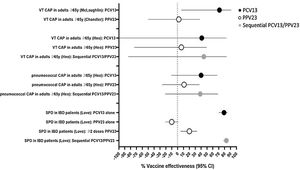
Four included studies evaluated VE against pneumococcal disease outcomes (Table 1). Three of these studies evaluated VE against vaccine-type CAP using a serotype-specific urinary antigen detection assay to facilitate case detection (Supplementary Table 1).11–13 In paired studies conducted in Louisville, United States, PCV13 displayed significant protection against PCV13-serotype CAP, whereas PPV23 did not demonstrate protection against PPV23-serotype CAP (Fig. 2; Supplementary Table 1).11,12 Minor differences in the conduct of the PPV23 study compared with the PCV13 study, such as the exclusion of patients who received PCV13 and differences in data sources for vaccination status, might have affected results. In a single study conducted in Korea, VE was estimated for vaccine-type CAP and pneumococcal CAP (any serotype), for the overall study population (≥65 years) and for two age-stratified subgroups (65–74 years, and ≥75 years).13 For vaccine-type CAP, no significant protection was observed for either vaccine, however the study was underpowered for this outcome (Fig. 2; Supplementary Table 1). For pneumococcal CAP caused by any serotype, no significant protection was observed in the overall (≥65 years) study population or in the ≥75 years age group (Supplementary Table 1). In the 65–74 years age group, PCV13 alone and sequential PCV13/PPV23 displayed protection against pneumococcal CAP, whereas PPV23 alone did not (Fig. 2; Supplementary Table 1). VE was also estimated for nonbacteremic pneumococcal CAP; as 161/167 (96.4%) of pneumococcal CAP cases were nonbacteremic, results were similar to those for pneumococcal CAP (Supplementary Table 1).
Vaccine effectiveness against pneumococcal disease outcomes. Results are from studies on pneumococcal community-acquired pneumonia (CAP) in hospitalized adults aged ≥65 years and severe pneumococcal disease (SPD) in inflammatory bowel disease (IBD) patients aged ≥18 years, described in Table 1. For vaccine-type CAP (VT CAP), PCV13 VE assessed PCV13-type CAP, PPV23 VE assessed PPV23-type CAP, and sequential PCV13/PPV23 VE assessed CAP caused by either PCV13 or PPV23 serotypes.
Love et al. investigated effectiveness of pneumococcal vaccination in a cohort of United States veterans aged ≥18 years with inflammatory bowel disease.14 In this cohort, 93% were male, the average age was 59 years, and patients were followed for an average of 5.4 years. The outcome of severe pneumococcal disease included IPD and pneumococcal pneumonia, however most (84.1%) cases were IPD. Pneumococcal serotypes were not reported. Both PCV13 alone and sequential PCV13/PPV23 demonstrated ∼80% VE against severe pneumococcal disease (Fig. 2; Supplementary Table 1). For PPV23, no effectiveness was observed in those who received ≥1 dose, however VE of 20% was found in participants who received ≥2 doses of PPV23.
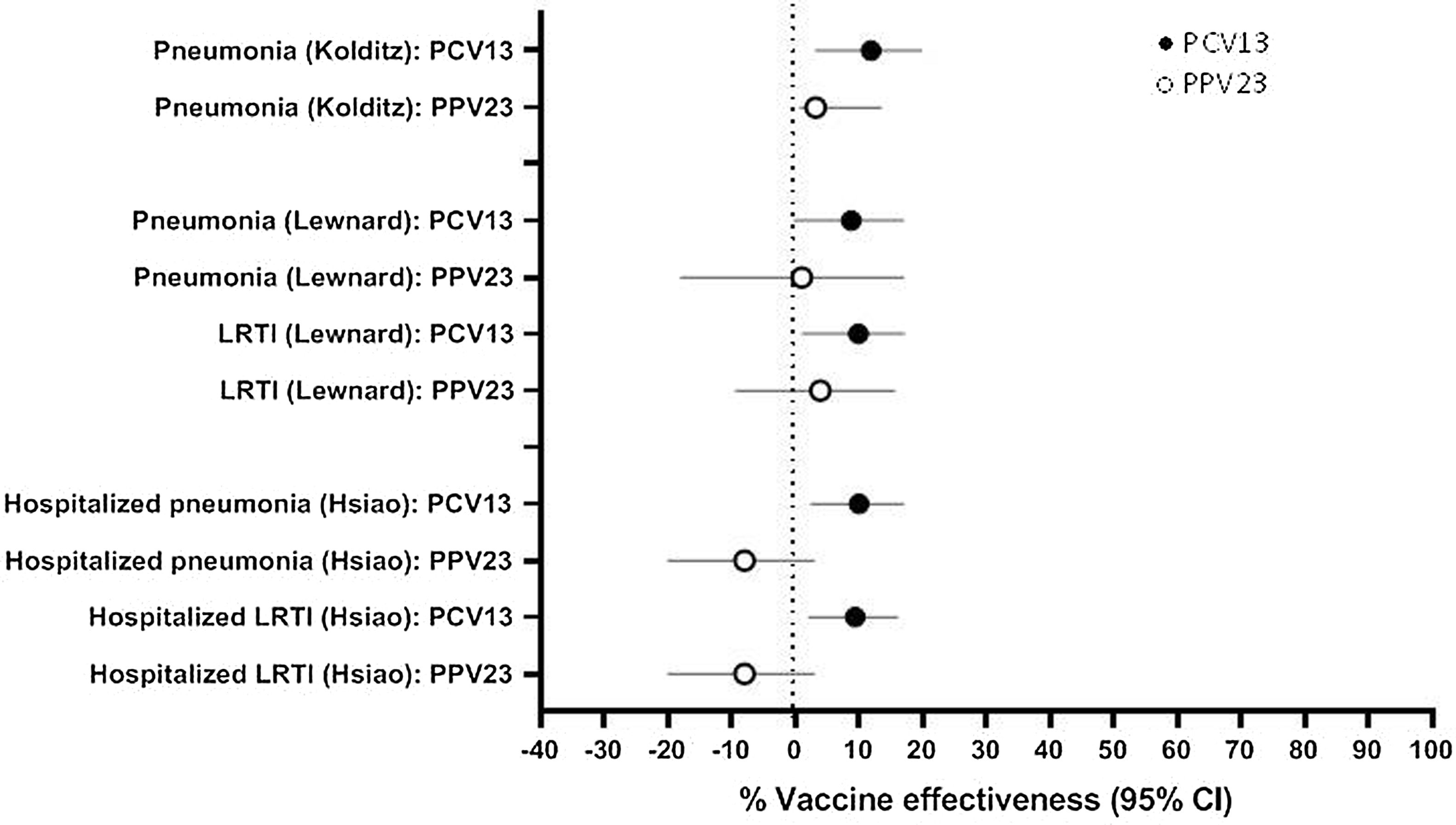
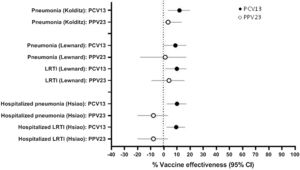
Pneumonia and LRTI OutcomesFour studies examined VE against clinically-defined respiratory outcomes including all-cause pneumonia and LRTI (Table 1). In Saxony, Germany, PPV23 was recommended for use in adults aged ≥60 years until 2011 and the recommendation changed to PCV13 in 2012.15,16 Kolditz et al. investigated pneumococcal VE against all-cause pneumonia during these two time frames, and found VE estimates of 3.2% and 11.9% for PPV23 and PCV13, respectively (Fig. 3; Supplementary Table 1).17,18 In both studies, subgroup analysis by sex found that significant protection was only observed in women (Supplementary Table 1). Lewnard and colleagues investigated the effectiveness of PCV13 and PPV23 against all-cause pneumonia and LRTI in older adults in southern California using a self-matched framework, comparing respiratory episodes for each participant before and after vaccination.19 PCV13 VE was 8.8% against pneumonia and 9.9% against LRTI, whereas PPV23 VE was 1.0% against pneumonia and 3.9% against LRTI (Fig. 3; Supplementary Table 1). Similar results were reported by Hsaio et al., who evaluated the effectiveness of PCV13 and PPV23 against hospitalized, all-cause pneumonia and LRTI in northern California using healthcare system data.20 PCV13 VE against pneumonia or LRTI ranged from 9 to 10%, whereas no effectiveness for PPV23 was observed (Fig. 3; Supplementary Table 1).
Vaccine effectiveness against all-cause pneumonia and all-cause lower respiratory tract infection (LRTI). Results are from studies in older adults conducted in Germany (Kolditz), southern California, United States (Lewnard), and northern California, United States (Hsiao) described in Table 1. Outcomes for the Hsiao study included hospitalized cases only; the other studies included inpatient and outpatient cases.
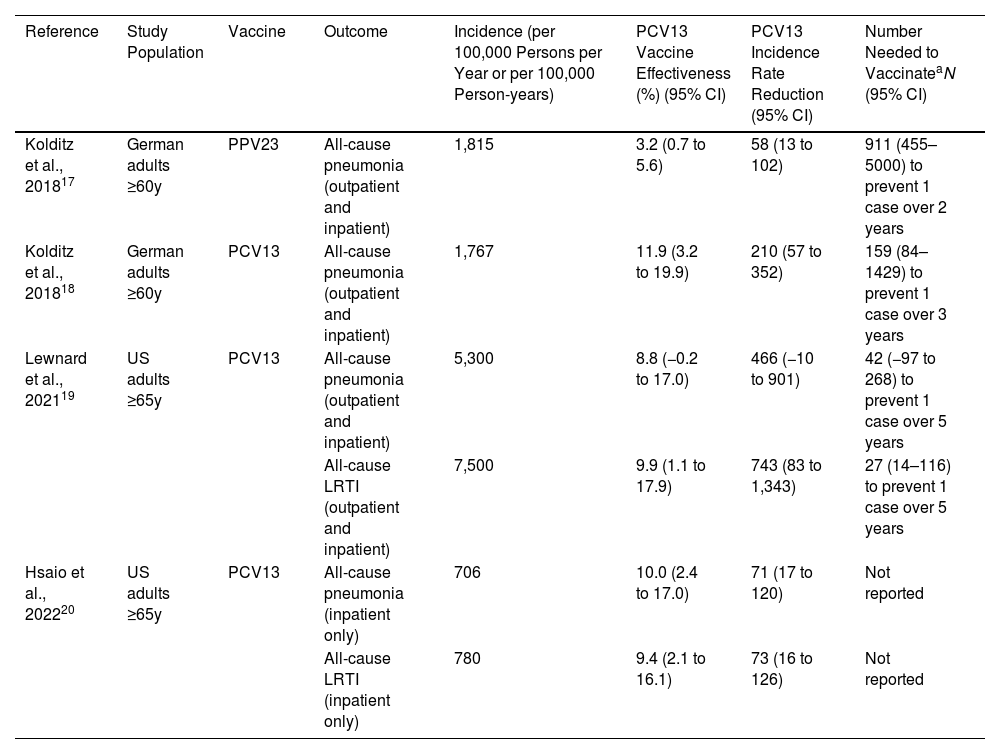
We calculated the IRR for studies that demonstrated significant effectiveness against all-cause pneumonia or LRTI (Table 2). In studies that assessed outpatients and inpatients, IRRs for PCV13 ranged from 210 to 743 per 100,000 persons/year or person-years, with smaller IRRs when only hospitalizations were assessed. In Germany, IRR for PPV23 was of 58 per 100,000 persons/year compared with 210 per 100,000 persons/year for PCV13. When reported, we included the estimated number needed to vaccinate to prevent an episode of all-cause pneumonia or LRTI in Table 2.
Incidence Rate Reduction for All-cause Pneumonia and All-cause Lower Respiratory Tract Infection (LRTI) for Studies in Which PCV13 or PPV23 Demonstrated Significant Effectiveness.
| Reference | Study Population | Vaccine | Outcome | Incidence (per 100,000 Persons per Year or per 100,000 Person-years) | PCV13 Vaccine Effectiveness (%) (95% CI) | PCV13 Incidence Rate Reduction (95% CI) | Number Needed to VaccinateaN (95% CI) |
|---|---|---|---|---|---|---|---|
| Kolditz et al., 201817 | German adults ≥60y | PPV23 | All-cause pneumonia (outpatient and inpatient) | 1,815 | 3.2 (0.7 to 5.6) | 58 (13 to 102) | 911 (455–5000) to prevent 1 case over 2 years |
| Kolditz et al., 201818 | German adults ≥60y | PCV13 | All-cause pneumonia (outpatient and inpatient) | 1,767 | 11.9 (3.2 to 19.9) | 210 (57 to 352) | 159 (84–1429) to prevent 1 case over 3 years |
| Lewnard et al., 202119 | US adults ≥65y | PCV13 | All-cause pneumonia (outpatient and inpatient) | 5,300 | 8.8 (−0.2 to 17.0) | 466 (−10 to 901) | 42 (−97 to 268) to prevent 1 case over 5 years |
| All-cause LRTI (outpatient and inpatient) | 7,500 | 9.9 (1.1 to 17.9) | 743 (83 to 1,343) | 27 (14–116) to prevent 1 case over 5 years | |||
| Hsaio et al., 202220 | US adults ≥65y | PCV13 | All-cause pneumonia (inpatient only) | 706 | 10.0 (2.4 to 17.0) | 71 (17 to 120) | Not reported |
| All-cause LRTI (inpatient only) | 780 | 9.4 (2.1 to 16.1) | 73 (16 to 126) | Not reported |
One included study examined the effectiveness of PCV13 and PPV23 against COVID-19 outcomes early during the pandemic, using administrative data from a cohort of southern California adults aged ≥65 years (Table 1).21 VE against hospitalized COVID-19 was 32% (95% CI, 17–43%) for PCV13 and 46% (95% CI 27–59%) for sequential PCV13/PPV23. In contrast, PPV23 VE against hospitalized COVID-19 was −2% (95% CI −29 to 27%). Results for COVID-19 diagnosis as an outcome were similar (Supplementary Table 1).
DiscussionTo our knowledge, this is the first review that compared PCV13 and PPV23 when evaluated in observational studies within a similar analysis framework. Within all included studies, point estimates for PCV13 VE were higher compared with PPV23 VE for all outcomes assessed. PCV13 consistently demonstrated protection against pneumococcal disease outcomes, all-cause pneumonia, all-cause LRTI, and in a single study evaluating COVID-19. In contrast, PPV23 VE was variable, with no significant protection observed in most studies. While sequential PCV13/PPV23 vaccination demonstrated protection against pneumococcal disease and broader respiratory outcomes, VE estimates were similar to those for PCV13 alone, although data were limited. This finding may be of interest for next-generation PCVs, as PCV15 has been recommended in sequence with PPV23 in the United States as an alternative to PCV20 alone.2
Several reasons could explain why PCV13 displayed higher effectiveness despite PPV23 containing more serotypes, such as differences in immune responses between plain polysaccharide and conjugated antigens, including induction of a T-cell-dependent response and immunologic memory by the conjugate vaccine.22 Importantly, PCVs but not PPV23 elicit mucosal immune responses that inhibit colonization of vaccine-type pneumococci.23 Protection against vaccine-type pneumococcal colonization by PCV13 has been demonstrated in adults using a human challenge model.24,25 It is plausible that a vaccine that inhibits pneumococcal colonization can also protect against pneumonia, which is largely a mucosal disease. Using a systems serology approach, Davies et al. compared humoral responses from adults vaccinated with PCV13 or PPV23 and found that PCV13 generated greater antigenic breath and antibody persistence compared with PPV23.26 Differences in antibody profiles might contribute to increased protection against carriage and pneumonia mediated by PCV13.
We did not conduct a meta-analysis due to differences in study designs, populations, and outcomes. As RCTs are considered the gold standard for vaccine evaluations, we explored whether they support or refute the findings presented here. For PCV13, evidence stems from one large trial conducted in healthy adults aged ≥65 years in the Netherlands, whereas for PPV23 several trials of moderate size have been conducted.27,28 For PCV13, results from the included observational studies were generally consistent with efficacy data, although confidence intervals were wide for some outcomes. For vaccine-type CAP, PCV13 VE ranged from 41 to 71%; although results from the Heo et al. study were not significant, these point estimates were in line with 45.6% efficacy against PCV13-type CAP.11–13,28 For pneumococcal pneumonia (any serotype), the Netherlands trial found PCV13 efficacy of 30.6%, consistent with VE against pneumococcal pneumonia reported by Heo et al.13,28 For PPV23, results were more variable. PPV23 VE against PPV23-type CAP ranged from 2 to 6.3% in the two included studies, contrasting with an RCT conducted in Swedish adults that found PPV23 efficacy of 79% (95% CI, −77 to 98) against PPV23-type pneumonia.12,13,27,29 However, the Swedish trial was conducted prior to pediatric PCV use and cases were restricted to bacteremic pneumonia, whereas cases from the observational studies were predominantly non-bacteremic, making comparisons difficult. Falkenhorst et al. reported pooled PPV23 efficacy against pneumococcal pneumonia from two clinical trials as 64% (95% CI 35–80), which contrasts to the lack of effectiveness of PPV23 found by Heo et al.11,30 However, the Falkenhorst pooled estimate was weighted 95.8% on an RCT conducted by Maruyama et al. among nursing home residents in Japan, a trial for which validity of the high VE estimate for pneumococcal pneumonia has been questioned.30–33
Our review uncovered consistent evidence that PCV13 provides protection against all-cause pneumonia and LRTI, with VE ∼10% across multiple studies. In contrast, for PPV23, only one of three included studies demonstrated significant effectiveness against all-cause pneumonia.17,19,20 The mechanisms by which PCV13 protects against all-cause respiratory infections are not known, and might reflect underdetection of pneumococcus by standard diagnostic methods.34 Another hypothesis is that PCV13 vaccination might influence synergistic interactions between pneumococci and respiratory viruses, leading to reductions in viral-associated disease.35 This hypothesis is supported by the study from Lewnard et al. showing that prior vaccination with PCV13 was associated with reduced risk of COVID-19 outcomes.21 A recent case–control study found that PCV13 was associated with reduced risk of viral LRTI in adults, with significant protection seen for several viruses including influenza and endemic human coronaviruses.36
Observational studies can provide useful real-world evidence of the benefits of vaccination, but are subject to bias. All included studies attempted to reduce bias, for example through propensity score matching,17,18 use of a test-negative design,11–13 or a self-matched framework.19 As both cases and controls in test-negative design studies were hosptalized for CAP, risk of bias due to use of medical care services is reduced, however results might not be generalizable to patients treated in the community. Additionally, other biases like residual ‘healthy vacinee’ effects might remain.37 All included studies had a ‘moderate risk’ of bias using the ROBINS-I tool, an expected result since ‘low risk’ of bias is equivalent to a high-quality randomized trial and rarely achieved by observational studies.9 By restricting our analysis to studies performed in the same population using consistent methods, vaccine comparisons should be mostly unaffected by differences in participant characteristics, serotype distribution, outcome and vaccine exposure definitions and the degree of misclassification.
Our review is subject to several limitations. No included studies assessed IPD alone or vaccine-type IPD. All included studies were conducted in high-income countries with mature infant PCV programs, so results might not be generalizable to other settings. While indirect effects and serotype replacement from pediatric PCV programs likely influenced effectiveness estimates for adult pneumococcal vaccines, IPD surveillance data indicate that indirect effects reach a plateau after initial declines in vaccine-type disease.38 Importantly, the time-to-follow up after vaccination varied across studies and was usually not specified, although most included studies limited their vaccinated population to people who received a vaccine within the previous five years.39 Only McLaughlin et al. reported the average length of follow up for PCV13-vaccinated or PPV23-vaccinated participants (157 and 553 days, respectively), however this paper only assessed VE for PCV13.11
Differences between PPV23 and PCV13 are likely to be a class effect and not specific to PCV13. Our findings might be useful for vaccine policy considerations, as they support use of PCVs in adults. In countries where PCV15 or PCV20 is licensed for adults, local data on serotype distribution in pneumococcal disease and cost-effectiveness analyses can inform decisions regarding the use of these vaccines alone or in combination with PPV23. In conclusion, in observational studies that assessed PCV13 and PPV23 in the same or similar populations, PCV13 consistently displayed greater VE compared with PPV23 for the outcomes assessed. Growing evidence indicates that adult immunization with PCV13 provides protection against all-cause respiratory infections in addition to confirmed pneumococcal disease. Higher-valency PCVs might have greater impact on a range of pneumococcal syndromes and all-cause pneumonia, a hypothesis that can be evaluated in post-licensure studies. Future studies evaluating the effectiveness of expanded-valency PCVs in real-world settings will be important to understating their full public health value.
FundingThis work was supported by Pfizer, Inc.
Authors’ ContributionsBDG conceived of the review with input from CT and LJ. EMD developed review methods with assistance from CT, CVM, CC, LRG, and BDG. EMD conducted the literature search, extracted data, and drafted the manuscript. CC, CVM, JL, LRG, MPES, LJ, CT, and BDG contributed to interpretation of data and edited the manuscript. All authors approved the final manuscript.
Conflicts of InterestEMD, LRG, LJ, CT, and BDG are employees of Pfizer, and as such, they may hold company stock. Pfizer was involved in the design, conduct, and writing of this collaborative review. MPES has received personal fees from GSK, Pfizer, AstraZeneca, and Sanofi Pasteur as a speaker at international meetings and as a member of advisory boards (unrelated to the submitted work). MPES has undertaken contract work for Pfizer.