In lung transplantation (LT), the length of ischemia time is controversial as it was arbitrarily stablished. We ought to explore the impact of extended cold-ischemia time (CIT) on ischemia-reperfusion injury in an experimental model.
MethodsExperimental, randomized pilot trial of parallel groups and final blind analysis using a swine model of LT. Donor animals (n=8) were submitted to organ procurement. Lungs were subjected to 6h (n=4) or 12h (n=4) aerobic hypothermic preservation. The left lung was transplanted and re-perfused for 4h. Lung biopsies were obtained at (i) the beginning of CIT, (ii) the end of CIT, (iii) 30min after reperfusion, and (iv) 4h after reperfusion. Lung-grafts were histologically assessed by microscopic lung injury score and wet-to-dry ratio. Inflammatory response was measured by determination of inflammatory cytokines. Caspase-3 activity was determined as apoptosis marker.
ResultsWe observed no differences on lung injury score or wet-to-dry ratio any given time between lungs subjected to 6h-CIT or 12h-CIT. IL-1β and IL6 showed an upward trend during reperfusion in both groups. TNF-α was peaked within 30min of reperfusion. IFN-γ was hardly detected. Caspase-3 immunoexpression was graded semiquantitatively by the percentage of stained cells. Twenty percent of apoptotic cells were observed 30min after reperfusion.
ConclusionsWe observed that 6 and 12h of CIT were equivalent in terms of microscopic lung injury, inflammatory profile and apoptosis in a LT swine model. The extent of lung injury measured by microscopic lung injury score, proinflammatory cytokines and caspase-3 determination was mild.
En el trasplante de pulmón (TP), la duración del tiempo de isquemia es controvertida, ya que se estableció de forma arbitraria. Sería útil explorar el impacto del tiempo de isquemia fría (TIF) prolongado sobre la lesión de isquemia-reperfusión en un modelo experimental.
MétodosEnsayo piloto experimental aleatorizado de grupos paralelos y análisis ciego final utilizando un modelo de TP en cerdos. Se extrajeron los órganos de los animales donantes (n=8). Los pulmones se conservaron durante 6 horas (n=4) o 12 horas (n=4) en hipotermia aeróbica. El pulmón izquierdo se trasplantó y reperfundió durante 4 horas. Se obtuvieron biopsias de pulmón (i) al comienzo del TIF, (ii) al final del TIF, (iii) 30 minutos después de la reperfusión y (iv) 4 horas después de la reperfusión. Los injertos de pulmón se evaluaron histológicamente mediante la puntuación de daño histológico pulmonar y la relación de peso húmedo y peso seco. La respuesta inflamatoria se valoró mediante la determinación de citoquinas inflamatorias. Se determinó la actividad de caspasa-3 como marcador de apoptosis.
ResultadosNo observamos diferencias en la puntuación de daño histológico pulmonar o en la relación de peso húmedo y peso seco en un momento dado entre los pulmones sometidos a 6 h-TIF o 12 h-TIF. Las IL-1β e IL-6 mostraron una tendencia ascendente durante la reperfusión en ambos grupos. El TNF-α alcanzó su punto máximo dentro de los 30 minutos posteriores a la reperfusión. Apenas se detectó IFN-γ. La inmunoexpresión de caspasa-3 se clasificó semicuantitativamente por el porcentaje de células teñidas. Se observó un 20% de células apoptóticas 30 minutos después de la reperfusión.
ConclusionesObservamos que 6 y 12 horas de TIF fueron equivalentes en términos de daño histológico pulmonar, perfil inflamatorio y apoptosis en un modelo de TP en cerdos. La extensión de la lesión pulmonar, medida por la puntuación de daño histológico pulmonar, las citoquinas proinflamatorias y la determinación de caspasa-3 fue leve.
Lung transplantation (LT) is the mainstay for treatment for patients with end-stage lung disease. Unfortunately, the shortage of suitable donors for transplantation remains a limiting factor and a challenge.1 Indeed, in most multiorgan-donors lungs are too deteriorated for donation and only 20–25% are acceptable for LT.2 Hence, it is critical to develop new strategies to increase the pool of organ donors. In this context, several alternatives have been investigated in recent years, including the use of so-called extended criteria donor lungs, the use of donors after cardiocirculatory death, and the implementation of ex vivo lung perfusion.3–5
Extending the accepted graft ischemia-time may increase the utility of donor lungs by enlarging the time frame to perform long distance lung procurements. Cold preservation has been the classic procedure to reduce lung injury while the organ is transported from donor to recipient. During lung procurement, lungs are flushed with a low potassium dextran solution, inflated with oxygen and stored at 4°C. The objective of hypothermic organ preservation is to decrease the cellular metabolism of the graft and thus, reduce the cell death ratio. However, ischemic insult leads to the generation of reactive oxygen species that result in cell injury.6 In this respect, it is worth noting that the limit of cold ischemia time (CIT) is poorly defined. In clinical LT, 8h is the generally accepted upper limit for CIT. Accordingly, extending the arbitrarily established ischemia time is a matter of debate because of the perceived increased rate of ischemia-reperfusion (IR) injury that could lead to primary graft disfunction (PGD).7,8
In the early post-operative period, PGD is the leading cause of morbidity and mortality, with up to 20% of recipients developing severe PGD. An important feature of PGD is the early development of inflammation as the result of the activation of innate immune mechanisms. These mechanisms involve the activation of pro-inflammatory transcription factors that induce the expression of inflammatory mediators, primarily inflammatory cytokines and chemokines, which promote the recruitment of activated leukocytes and subsequent inflammatory cytotoxic consequences.7–9 The mechanisms responsible for the triggering of innate immune response and acute inflammation in IR injury have not been elucidated so far. In this respect, several clinical series have demonstrated that the perceived detrimental effect of extended graft ischemia time on PGD might not be well founded.10–12
In view of the limited evidence in the clinical setting, the objective of the present study was to analyze the impact of extended CIT on IR injury following LT in a swine experimental model by means of determination of histologic injury, inflammatory profile and cell death in the lung graft.
MethodsThis was an experimental, randomized pilot trial of parallel groups and a final blind analysis using a swine model of LT developed for the purpose of the study. Animals received humane care during experiments in compliance with the “The Guide for the Care and Use of Laboratory Animals” and were housed and handled in accordance with European law (Directive 2010/63/EU). The study was evaluated and approved by hospital and competent authority's Ethics Committee on Animal Research (no. 10325). Sixteen female commercial hybrid pigs weighing 30–35kg were used (8 as donors and 8 as receptors).
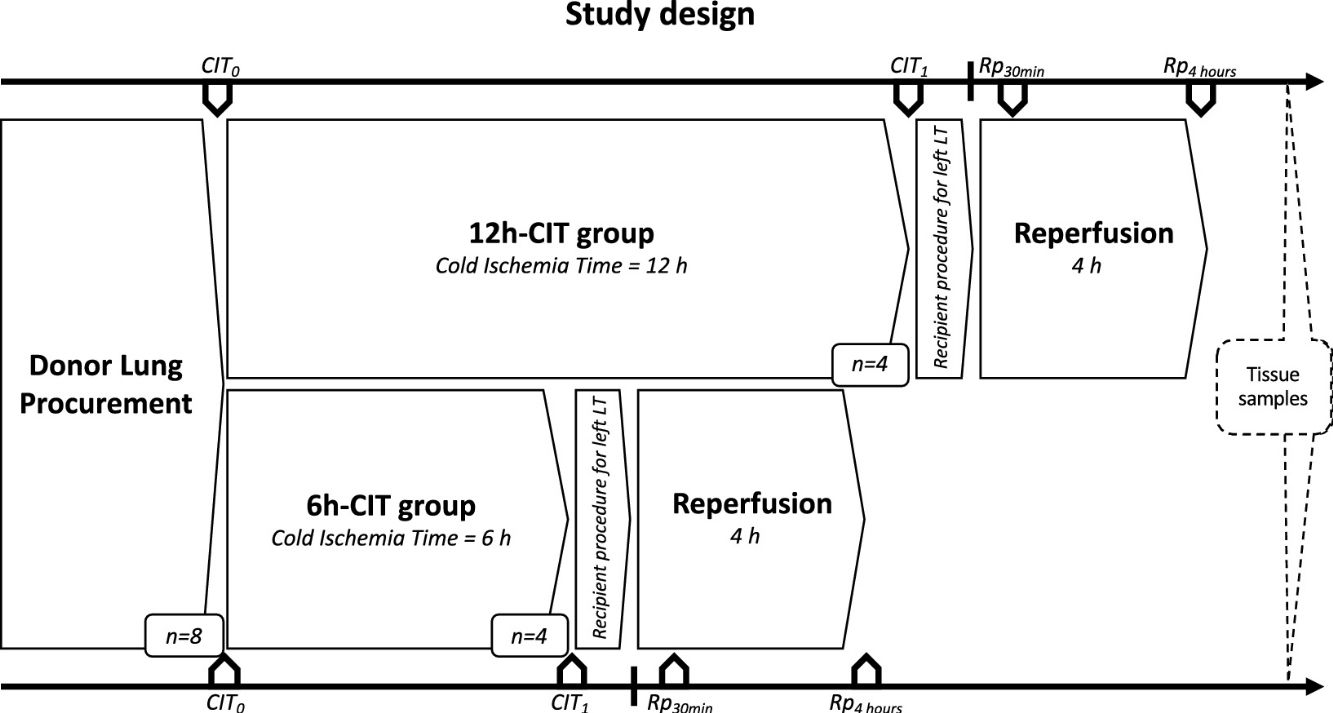
Study designDonor animals (n=8) were submitted to organ procurement under general anesthesia. The lungs were separated into two groups, the 6h-CIT group (n=4) and the 12h-CIT group (n=4) and were respectively subjected to 6h and 12h of ischemic aerobic hypothermic static preservation. At the end of the preservation, the left lung was transplanted into a recipient animal and re-perfused for 4h (Fig. 1).
Study design. This study was comprised of 2 groups (n=4 each). Swine donor lungs were preserved at 4°C for 12h in the 12h-CIT group, and for 6h in the 6h-CIT group, respectively. The left lungs were transplanted and reperfused for 4h. Lung tissue samples were collected at different timepoints as follows: (i) CIT0: at the beginning of the CIT, (ii) CIT1: at the end of the CIT; (iii) Rp30min: 30min after the reperfusion of the left LT; (iv) Rp4h: 4h after the reperfusion of the left LT. CIT: cold ischemia time; LT: lung transplant.
Pigs were sedated with ketamine (8mg/kg im), midazolam (0.6mg/kg im), and xylazine 20% (2.2mg/kg im). Induction was carried out in the operating room with inhaled sevoflurane 4% with 1L/min oxygen via face mask. Cefazolin (20mg/kg iv) and methylprednisolone (500mg iv) were administered after induction. Pigs were cannulated with an endotracheal tube. Maintenance agents consisted of propofol (5–8mg/kg/h iv) and remifentanil (2–20mcg/kg/h iv) infusions. Animals were ventilated with a pressure-controlled ventilator at 15cmH2O, a tidal volume of 6–8ml/kg, positive end-expiratory pressure (PEEP) of 5cmH2O, with a respiratory rate of 15breaths/min, and a FiO2 of 0.5. Median sternotomy was performed and the superior and inferior vena cava were encircled with silk ties. A bolus of heparin 10.000U/ml was injected intravenously. A 21 French cannula was inserted into the main pulmonary artery (PA). A bolus of 500mcg prostaglandin E1 was injected in the main PA. The superior and inferior cava were ligated, a clamp was placed across the aorta and the left atrial appendage was transected. The PA was flushed with Perfadex Plus (60ml/kg) solution at 4°C. The trachea was stapled with the lungs inflated with a sustained airway pressure of 15cmH2O. A retrograde flush through the left atrium with 15ml/kg of Perfadex Plus solution was performed. The heart-lung bloc was placed in a plastic bag containing 500ml of Perfadex Plus at 4°C.
Lung transplant procedureThe recipient pigs were sedated and anesthesized in the same way as the donors. A left thoracotomy was performed through the fourth intercostal space. The left azygous vein was ligated and the inferior pulmonary ligament divided. A vascular clamp was placed proximally on the left main PA which was divided distally. The left pulmonary veins were ligated and divided. Then, the bronchus was divided. Bronchial anastomosis was performed with a running 4/0 Prolene suture, PA anastomosis with a continuous 5/0 Prolene suture and then atrial anastomosis with a running 5/0 Prolene suture. After re-inflation of the transplanted lung to a pressure of 20–25cmH2O, the PA clamp was removed gradually and the lung was de-aired through the left atrial anastomosis. The lungs were then ventilated with 60% oxygen, 5cm H2O PEEP and the respiratory rate was adjusted to keep the pCO2 between 35 and 40mmHg.
Allograft tissue sample collectionLung tissue biopsies were collected from the left lower lobe at the following time points: (a) CIT0: at the beginning of the CIT, (b) CIT1: at the end of the CIT; (c) Rp30min: 30min after reperfusion of the left LT; (d) Rp4h: 4h after reperfusion of the left LT. Half of the biopsy was stained with hematoxylin–eosin, whereas the other half was flash frozen in liquid nitrogen and stored at −80°C.
Histologic assessment of microscopic lung injury and lung edemaA pulmonary pathologist calculated the histopathologic grading of acute lung injury according to the following parameters: interstitial edema, intra-alveolar edema, hemorrhage, cell infiltration and hyaline membrane formation.13 The severity of the findings was graded on a scale from 0 (absent) to 3 (severe). The lung injury score developed on this basis is shown in Table 1.
Histopathological assessment by lung injury score.
| Lung injury scale | |
|---|---|
| Parameter | Points |
| Interstitial edema | 0–3 |
| Alveolar edema | 0–3 |
| Hemorrhage | 0–3 |
| Cell infiltration | 0–3 |
| Hyaline membrane formation | 0–3 |
| Lung injury score | |
|---|---|
| Points | Score |
| 0 | No damage |
| 1–5 | Mild damage |
| 6–10 | Moderate damage |
| 11–15 | Severe damage |
The wet-to-dry weight ratio was used to evaluate lung edema by measuring the degree of water in the lung.
Lung tissue homogenates preparation and ELISA for cytokinesThe inflammatory response was analyzed in lung tissue samples stored at −80°C. Lung tissue cytokines IFN-γ, IL-1β, IL6, IL10 and TNF-α were evaluated with the Cytokine & Chemokine 9-Plex Porcine ProcartaPlex™ Panel 1 (ThermoFisher Scientific, Bender MedSystems GmbH, Vienna, Austria). Cytokine concentrations were calculated using the ProcartaPlex Analyst software.
Apoptotic cell death pathway, caspase-3 in tissueThe detection of activated caspase 3 provided a sensitive means of detecting apoptotic cells. Endogenous peroxidase was blocked before staining the sections. Antibodies against cleaved caspase-3 (Asp175) (1:100, Cell Signaling Technology, United States) were used. Immunoexpression was graded semiquantitatively by considering the percentage of stained cells. A percentage score was obtained from each sample applying the following formula: (stained cells in the slide/total cells in the slide)×100.
Statistical methodsAbsolute frequencies and percentages were used to describe the qualitative variables. Quantitative variables were described using the mean, standard deviation (SD), median and quartiles. The Kolmogorov–Smirnov test was used to assess the normality of distributions. The variables, weight (kg), PO2 (mmHg) and warm ischemia time (WIT) (min), were compared between study groups (6h vs 12h) in donor and recipient animals using the U Mann–Whitney test. Differences between study groups in cytokine levels at different timepoints were analyzed using the Mann–Whitney test. Cytokine patterns in each group during the experiment were also analyzed using the Friedman test. For all the tests, P-values of <0.05 were considered statistically significant. The statistical package R Studio (2.5) was used for the statistical analyses.
ResultsBaseline characteristicsNo statistically significant differences were found between the pigs subjected to 6h or 12h CIT in the operative variables of, donor/recipient body weight, donor/recipient partial pressure of arterial oxygen (PO2) before thoracotomy and WIT (Table 2).
Donor and recipient baseline characteristics.
| Variable | Statistic | Total (n=8) | 6h (n=4) | 12h (n=4) | P-valuea |
|---|---|---|---|---|---|
| Donor | |||||
| Weight (kg) | Mean (SD) | 31.31 (3.21) | 30.38 (3.54) | 32.25 (3.01) | 0.306 |
| Median (Q1; Q3) | 30.25 (28.5; 34.75) | 29 (28; 32.75) | 32.25 (29.75; 34.75) | ||
| PO2 (mmHg) | Mean (SD) | 488.38 (92.46) | 443 (82.31) | 533.75 (87.65) | 0.149 |
| Median (Q1; Q3) | 506 (412; 567) | 450.5 (380; 506) | 567 (485; 582.5) | ||
| Recipient | |||||
| Weight (kg) | Mean (SD) | 34.25 (3.18) | 33.88 (3.75) | 34.63 (3.04) | 0.663 |
| Median (Q1; Q3) | 33.5 (32; 37) | 33.25 (31.5; 36.25) | 34.75 (32.25; 37) | ||
| PO2 (mmHg) | Mean (SD) | 460.88 (107.28) | 471.75 (60.26) | 450 (151.36) | 1.000 |
| Median (Q1; Q3) | 467 (420; 526) | 467 (420; 523.5) | 469.5 (337; 563) | ||
| WIT (min) | Mean (SD) | 91.88 (11.32) | 90.25 (6.29) | 93.5 (15.89) | 0.564 |
| Median (Q1; Q3) | 93 (85; 100.5) | 91 (85; 95.5) | 98 (81.5; 105.5) | ||
WIT: warm ischemia time. Q1: first quartile; Q3, third quartile; SD, standard deviation.
Recipient’ monitoring parameters throughout the reperfusion period in the 6h-CIT and 12h-CIT groups are collected in the Appendix 1 (supplementary material).
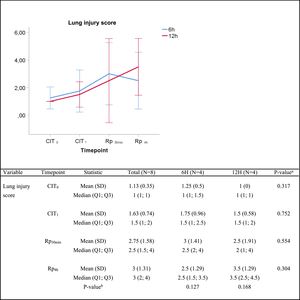
Histologic lung injury and pulmonary edemaIn the histologic assessment, lungs subjected to 6h or 12h static cold storage presented no statistically significant differences in the lung injury score at any given time, as follows: median lung injury score at (i) CIT0, 1 vs. 1 (P=0.317) for the 6h-CIT and 12h-CIT group, respectively; (ii) CIT1, 1.5 vs. 1.5 (P=0.752); (iii) Rp30min, 2.5 vs. 2 (P=0.659) and; (iv) Rp4h, 2.5 vs. 3.5 (P=0.304). In all cases, the tissue obtained 4h after lung reperfusion showed greater tissue injury (score rank 1–5) than the corresponding sample at the end of the cold ischemia (score rank 1–3). However, in both situations the score corresponded to a mild pulmonary lesion (Fig. 2). The histological analysis revealed a case of pneumonia in one lung corresponding to the 6h-CIT group. In this particular graft, mild (corresponding to timepoint CIT0) to severe (corresponding to CIT1, Rp30min and Rp4h) cellular inflammation was identified in hematoxylin & eosin (H&E) staining. This fact was not noticed during the macroscopic inspection when performing the pulmonary procurement.
Lung injury score. Score 0: no pulmonary injury; score 1–5: mild pulmonary injury; score 6–10: moderate pulmonary injury and score 11–15: severe pulmonary injury. Q1: first quartile; Q3, third quartile; SD, standard deviation. CIT indicates cold ischemia time; CIT0, beginning of the CIT; CIT1, end of the CIT; Rp30min, 30min after the reperfusion of the left LT; Rp4h, 4h after the reperfusion of the left lung tranplant. aP-values obtained from the Mann–Whitney test (6h vs 12h). bP-values obtained from the Friedman test (CIT1 vs Rp30min vs Rp4h).
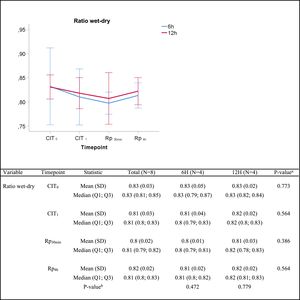
In the lung edema assessment, no significant differences were found in the wet/dry lung weight ratio between groups at any given time (Fig. 3).
Wet-to-dry ratio. Q1: first quartile; Q3, third quartile; SD, standard deviation. CIT: cold ischemia time; CIT0, beginning of the CIT; CIT1, end of the CIT; Rp30min, 30min after the reperfusion of the left LT; Rp4h, 4h after the reperfusion of the left lung tranplant. aP-values obtained from the Mann–Whitney test (6h vs 12h). bP-values obtained from the Friedman test (CIT1 vs Rp30min vs Rp4h).
Lung injury scale parameters throughout the experiment in 6h-CIT and 12h-CIT groups are collected in Appendix 2 (supplementary material).
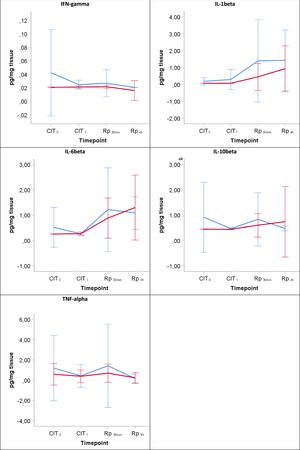
Inflammatory responseThroughout the experiment no significant differences were found between the groups corresponding to 6h and 12h of hypothermic static preservation in the ELISA analysis for the following inflammatory cytokines: IFN-γ, IL-1β, IL6, IL10 and TNF-α (Table 3, Fig. 4). There was a slight amount of IFN-γ detected during reperfusion, but its peak remained close to baseline values (median 0.02pg/mg). Among the proinflammatory cytokines, IL-1β and IL6 showed an upward trend during the reperfusion period. No significant differences in the regulatory cytokine IL10 were observed. TNF-α was barely detected during CIT (at CIT1, the median TNF-α quantity measured in pg/mg was 0.09 vs. 0.32 for 6h-CIT and 12h-CIT, respectively: P=0.773), but was clearly detected within 30min of reperfusion (at Rp30min, the median TNF-α quantity was 0.17 vs. 0.56 for 6h-CIT and 12h-CIT, respectively: P=0.386) and then returned to baseline values.
Inflammatory cytokines throughout the experiment in 6h-CIT and 12h-CIT groups.
| Variable | Timepoint | Statistic | Total (n=8) | 6h (n=4) | 12h (n=4) | P-valuea |
|---|---|---|---|---|---|---|
| IFN-γ pg/mg | CIT0 | Mean (SD) | 0.03 (0.03) | 0.04 (0.04) | 0.02 (0) | 0.767 |
| Median (Q1; Q3) | 0.02 (0.02; 0.02) | 0.02 (0.02; 0.06) | 0.02 (0.02; 0.02) | |||
| CIT1 | Mean (SD) | 0.02 (0) | 0.02 (0) | 0.02 (0) | 0.248 | |
| Median (Q1; Q3) | 0.02 (0.02; 0.02) | 0.02 (0.02; 0.03) | 0.02 (0.02; 0.02) | |||
| Rp30min | Mean (SD) | 0.02 (0.01) | 0.03 (0.01) | 0.02 (0) | 0.885 | |
| Median (Q1; Q3) | 0.02 (0.02; 0.02) | 0.02 (0.02; 0.03) | 0.02 (0.02; 0.02) | |||
| Rp4h | Mean (SD) | 0.02 (0.01) | 0.02 (0) | 0.02 (0.01) | 0.885 | |
| Median (Q1; Q3) | 0.02 (0.02; 0.02) | 0.02 (0.02; 0.02) | 0.02 (0.01; 0.02) | |||
| P-valueb | 0.282 | 0.627 | ||||
| IL-1β pg/mg | CIT0 | Mean (SD) | 0.13 (0.1) | 0.18 (0.13) | 0.07 (0) | 0.191 |
| Median (Q1; Q3) | 0.07 (0.07; 0.15) | 0.15 (0.08; 0.28) | 0.07 (0.07; 0.07) | |||
| CIT1 | Mean (SD) | 0.18 (0.27) | 0.29 (0.37) | 0.07 (0) | 0.149 | |
| Median (Q1; Q3) | 0.07 (0.07; 0.12) | 0.12 (0.07; 0.51) | 0.07 (0.07; 0.07) | |||
| Rp30min | Mean (SD) | 0.92 (1.17) | 1.39 (1.54) | 0.45 (0.5) | 0.564 | |
| Median (Q1; Q3) | 0.34 (0.08; 1.6) | 1.12 (0.13; 2.65) | 0.29 (0.08; 0.82) | |||
| Rp4h | Mean (SD) | 1.18 (0.96) | 1.43 (1.13) | 0.93 (0.85) | 0.564 | |
| Median (Q1; Q3) | 1.12 (0.43; 1.66) | 1.12 (0.61; 2.25) | 0.97 (0.2; 1.66) | |||
| P-valueb | 0.105 | 0.174 | ||||
| IL6pg/mg | CIT0 | Mean (SD) | 0.39 (0.35) | 0.52 (0.49) | 0.25 (0) | 0.767 |
| Median (Q1; Q3) | 0.25 (0.25; 0.29) | 0.28 (0.25; 0.79) | 0.25 (0.25; 0.26) | |||
| CIT1 | Mean (SD) | 0.27 (0.03) | 0.26 (0.02) | 0.27 (0.04) | 0.885 | |
| Median (Q1; Q3) | 0.25 (0.25; 0.28) | 0.26 (0.25; 0.28) | 0.25 (0.25; 0.29) | |||
| Rp30min | Mean (SD) | 1.05 (0.77) | 1.22 (1.04) | 0.88 (0.5) | 0.773 | |
| Median (Q1; Q3) | 0.84 (0.6; 1.25) | 0.84 (0.62; 1.82) | 0.91 (0.51; 1.25) | |||
| Rp4h | Mean (SD) | 1.19 (0.6) | 1.08 (0.41) | 1.3 (0.8) | 0.386 | |
| Median (Q1; Q3) | 1.24 (0.8; 1.7) | 1.08 (0.8; 1.36) | 1.58 (0.74; 1.85) | |||
| P-valueb | 0.050c,d | 0.174 | ||||
| IL10pg/mg | CIT0 | Mean (SD) | 0.68 (0.62) | 0.91 (0.87) | 0.45 (0.01) | 0.767 |
| Median (Q1; Q3) | 0.44 (0.44; 0.51) | 0.5 (0.44; 1.38) | 0.44 (0.44; 0.45) | |||
| CIT1 | Mean (SD) | 0.46 (0.02) | 0.47 (0.03) | 0.44 (0) | 0.468 | |
| Median (Q1; Q3) | 0.44 (0.44; 0.46) | 0.46 (0.44; 0.49) | 0.44 (0.44; 0.45) | |||
| Rp30min | Mean (SD) | 0.71 (0.49) | 0.83 (0.66) | 0.6 (0.29) | 0.554 | |
| Median (Q1; Q3) | 0.46 (0.44; 0.83) | 0.53 (0.45; 1.22) | 0.46 (0.44; 0.75) | |||
| Rp4h | Mean (SD) | 0.61 (0.59) | 0.47 (0.06) | 0.74 (0.87) | 0.885 | |
| Median (Q1; Q3) | 0.44 (0.44; 0.5) | 0.45 (0.44; 0.5) | 0.44 (0.24; 1.23) | |||
| P-valueb | 0.282 | 0.807 | ||||
| TNFα pg/mg | CIT0 | Mean (SD) | 0.89 (1.44) | 1.19 (2.03) | 0.58 (0.67) | 0.773 |
| Median (Q1; Q3) | 0.28 (0.03; 1.11) | 0.26 (0.03; 2.35) | 0.43 (0.05; 1.11) | |||
| CIT1 | Mean (SD) | 0.4 (0.53) | 0.42 (0.7) | 0.38 (0.4) | 0.773 | |
| Median (Q1; Q3) | 0.12 (0.03; 0.7) | 0.09 (0.03; 0.81) | 0.32 (0.06; 0.7) | |||
| Rp30min | Mean (SD) | 1.05 (1.78) | 1.42 (2.59) | 0.69 (0.56) | 0.386 | |
| Median (Q1; Q3) | 0.28 (0.16; 1.1) | 0.17 (0.09; 2.75) | 0.56 (0.28; 1.1) | |||
| Rp4h | Mean (SD) | 0.2 (0.28) | 0.16 (0.27) | 0.23 (0.33) | 0.191 | |
| Median (Q1; Q3) | 0.04 (0.03; 0.34) | 0.03 (0.03; 0.3) | 0.08 (0.04; 0.42) | |||
| P-valueb | 0.082 | 0.165 |
CIT: cold ischemia time; CIT0, beginning of the CIT; CIT1, end of the CIT; LT, lung transplant; Rp30min, 30min after the reperfusion of the left LT; Rp4h, 4h after the reperfusion of the left LT.
aP-value<0.05 obtained from the Mann–Whitney test (6h vs 12h).
The lines show the expression of proinflammatory cytokines in lung tissue throughout the experiment. CIT: cold ischemia time; CIT0, beginning of the CIT; CIT1, end of the CIT; LT, lung transplant; Rp30min, 30min after the reperfusion of the left LT; Rp4h, 4h after the reperfusion of the left LT. aP-value<0.05 obtained from the Mann–Whitney test (6h vs 12h). bP-value<0.05 obtained from the Friedman test (CIT1 vs Rp30min vs Rp4h). cP<0.05 for pairwise comparison CIT1 vs Rp30min; dP<0.05 for pairwise comparison CIT1 vs Rp4h; eP<0.05 for pairwise comparison Rp30min vs Rp4h.
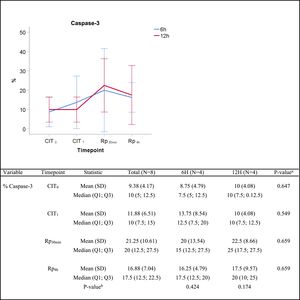
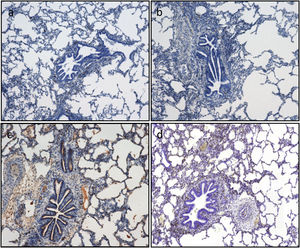
The semiquantitative analysis of caspase-3 immunostaining showed an increasing trend throughout the experiment. The median percentage of caspase-3 positive cells increased from 7.5% vs. 10% at timepoint CIT0 (P=0.647), to 15% vs. 25% at timepoint Rp30mim (P=0.659) for the 6h-CIT and 12h-CIT groups, respectively. Peak expression of caspase-3 was observed in samples corresponding to 30min after reperfusion of the transplanted lung. The increase was similar in the 6h-CIT and 12h-CIT groups (Fig. 5). The highest percentage of caspase-3 positive cells (40%) was found at timepoint Rp30min, corresponding to the 6h-CIT graft with pneumonia. Representative images of caspase-3 positive cell staining are shown in Fig. 6 in chronological order along the timepoints of the experiment.
Caspase-3 semiquantitative analysis. Q1: first quartile; Q3, third quartile; SD, standard deviation. CIT: cold ischemia time; CIT0, beginning of the CIT; CIT1, end of the CIT; Rp30min, 30min after the reperfusion of the left LT; Rp4h, 4h after the reperfusion of the left lung transplant. aP-values obtained from the Mann-Whitney test (6h vs 12h). bP-values obtained from the Friedman test (CIT1 vs Rp30min vs Rp4h).
Apoptotic cell death is assessed by immunohistochemistry quantification of caspase-3. Caspase-3 positive cells are stained in brown. Representative images (a)–(d). According to the semiquantitative analysis, the percentage of stained cells in lung tissue as follows: (a) 5% corresponding to timepoint CIT0; (b) 5% corresponding to timepoint CIT1; (c) 20% corresponding to timepoint Rp30min; and (d) 15% corresponding to timepoint Rp4h.
In this pilot swine model of LT after extended CIT, the performance of donor lungs that underwent 12h CIT was found to be as good as that of lungs that underwent 6h CIT. No differences were found in terms of decisive characteristics of acute lung injury14,15 such as microscopic lung injury, lung edema, inflammation or apoptosis.
The maximum safe preservation time for human LT remains undefined. However, there is a reluctance to extend the conventionally accepted <8h of cold ischemia.16 Thabut et al.16 analyzed data from 752 patients who underwent LT in seven French transplantation centers during a 12-year period. Graft ischemic time was associated with early PGD and with long-term survival in patients undergoing single or double LT, but not in patients undergoing heart-lung transplantation. More recently, a retrospective study based on United Network of Organ Sharing (UNOS) database evaluated the effect of prolonged total graft ischemia times (>6h) on long-term survival and the development of PGD.10 Interestingly, prolonged graft ischemia was not associated with an increase in 1 and 5-year mortality and was also not a negative predictor of PGF.
Bearing in mind that reproducibility in animal research and translation from bench to bedside is still a challenge,17 our large animal model study design emulated the clinical setting where lungs recovered for transplantation require a period of aerobic cold ischemic preservation to be transported back to the transplant center.
IR injury is inevitable in solid organ transplantation.18 However, the lung has to be considered differently because it contains oxygen in the alveoli which, helps to maintain aerobic metabolism and prevents hypoxia during ischemic preservation.19–21 In addition, ischemia is characterized by the absence of blood flow into the lung, which can cause oxidant injury despite the presence of oxygen. Thus, it can occur during the storage period.21,22 Our results with regards to microscopic lung injury deteriorated to a similar degree in both the 6h-CIT and 12h-CIT groups after reperfusion. Activation of the innate immune system induces the expression of inflammatory mediators which promote the recruitment of activated leukocytes that have subsequent inflammatory cytotoxic consequences.18,23,24 We assessed the development of graft inflammation through the evaluation of key proinflammatory cytokines. The exposure of lung cells to 6 and 12h of CIT followed by 4h of reperfusion resulted in enhanced proinflammatory cytokines such as TNF-α, IL-1β and IL-6. Interestingly, we observed that TNF-α protein was expressed early and then returned to baseline production at 4h of reperfusion. This early appearance of TNF-α supports the implication that it regulates the expression of other proinflammatory cytokines and affects neutrophil recruitment as early as 30min after reperfusion. We did not observe a significant IFN-γ response due to the short reperfusion period. It has been demonstrated that recipient T-cell activation occurs during the late phase of IR injury together with IFN-γ release in a lung transplant setting.25 Likewise, levels of IL-10, the only anti-inflammatory cytokine, remained barely detectable during the reperfusion period.
Unlike necrosis, apoptosis does not occur during ischemia, though it does increase during reperfusion.26 The induction of apoptosis can occur through the intrinsic or extrinsic pathway. The first is dependent on mitochondria and is activated by reactive oxygen species. The second is dependent on inflammatory molecules, such as TNF-α. In LT, it has been observed that apoptosis peaks rapidly after reperfusion corresponding to up to 30% cells of lung graft.27 In our study, caspase-3 activity was used as a hallmark indicator of apoptosis in LT.28 Although caspase-3 did increase during the reperfusion period, the increase was only slight and did not differ between the study groups. Peak expression of caspase-3 was observed in samples corresponding to 30min after reperfusion. Interestingly, this timepoint coincides with peak expression of TNF-α. In addition, our data suggest that the presence of apoptosis was not related with lung function in terms of edema development. Similar results have been reported in other studies in an experimental setting.29,30
This study has several limitations in the context of large animal experimental models. First, given its nature as a pilot study, the trial was designed as an initial test to analyze CIT in early ischemia-reperfusion injury. For this reason, it was conducted with a small number of subjects and, consequently, its statistical power to detect differences was compromised. Second, the reperfusion period was 4h, far below the 72 h-range covered by the clinical definition of PGD. However, we believe that this does not introduce significant bias as a large number of published works are limited to a 2–4-h reperfusion period.31–33 Because of the complexity of the swine LT model, the literature regarding 3 days survival after LT is scarce.34,35 Thirdly, one of the animals in the 6h-CIT group presented a pneumonia that was not detected macroscopically during lung procurement. Interestingly, this animal had the highest microscopic injury score and apoptotic cell death percentage. In fact, infection is a well-known risk factor for the development of PGD.36 The design of our study mirrors clinical practice where lungs recovered for transplantation require a period of cold ischemic preservation. However, bearing in mind the complex setting of the lung transplant procedure, a clinical trial with prolonged CITs would not be feasible for ethical reasons. It is therefore difficult to translate measures taken in an experimental context into clinical practice. In any case, because humans share more immune-system-related genes and proteins with pigs than rodents or other animals, the swine model is the most widely used large animal model in the context of lung transplantation. Although our study represents a proof of concept in pigs, its preliminary outcomes warrant further testing using a larger sample size with a longer-term survival model.
Nonetheless, there are also a number of noteworthy strengths in our study. To begin with, we believe that our work provides relevant information about the extension of the arbitrarily stablished ischemia time. Geographically distant organ procurement may be facilitated by increasing the viable time frame.
Our study additionally provides evidence to support a CIT of above 12h to obtain a model of moderate lung damage in the experimental setting. Using a histological lung injury score, as well as inflammation and apoptosis analyses, our study shows that 12h of CIT leads to mild damage. Thus, lungs that are completely healthy, or only mildly damaged, may not reflect the potential improvement provided by any tested treatment. Accordingly, the most recent experimental studies involving pig lung damage models apply CITs ranging from 18 to 24h.37–39 Finally, as our work precisely describes the technique of LT in pigs, recording not only the anesthetic and surgical protocols but also the technical information for sample processing, it consequently enables the reproducibility of these complex procedures.
In conclusion, we have demonstrated in a swine model that post-transplant lung allograft injury was equivalent in specimens that underwent 6h or 12h CIT. No statistically significant differences were found in lung injury markers between the two groups. The extent of lung injury measured using a microscopic lung injury score, proinflammatory cytokines and caspase-3 determination was mild. The implications that may arise from this study should be taken with caution due to its experimental character. Outcomes from this study warrant further testing using a bigger sample size and longer-term survival model to investigate the role of CIT in PGD.
Contributions(I) Conception and design: Ojanguren A; (II) Administrative support: Ojanguren A, Olsina-Kissler JJ; Provision of study materials: Ojanguren A, Milla L, Fraile-Olivero C, Puy S; Olsina-Kissler JJ; (IV) Collection and assembly of data: Ojanguren A, Milla L, Fraile C, Puy S, Santamaria M.; (V) Data analysis and interpretation: Ojanguren A, Gatius S, Gómez-Olles S, Boada-Pérez M, Esquinas C, Santamaria M; (VI): Manuscript writing: All authors; (VII); Final approval of manuscript: All authors.
DisclosureM.B. has received speaker fees from Grifols, Menarini, CLS Behring, GSK and Boehringer Ingelheim, and consulting fees from GSK, Novartis, Boehringer Ingelheim and GebroPharma, outside the submitted work.
I.O. has received travel grants, consulting fees, speaker fees, or research grants from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, MSD, GlaxoSmithKline, Menarini, Mundipharma, Novartis, and Teva, outside the submitted work.
Conflict of interestsThe authors declare that they have no conflict of interests related to this article.
This research was supported by funding to A.O. by Sociedad Española de Neumología y Cirugía Torácica (SEPAR), Societat Catalana de Pneumología (SOCAP), Sociedad Española de Cirugía Torácica (SECT), Institut de Recerca Biomèdica Lleida (IRBLleida) and Universitat de Lleida.
Work supported by Scientific and Technical Service of Immunohistochemistry, Lleida Institute for Biomedical Research Dr. Pifarré Foundation, IRBLleida.
I.O. is a researcher supported by the Pla Estratègic de Recerca i Innovació en salut (PERIS) 2016-2020 (SLT008/18/00108; G60594009).