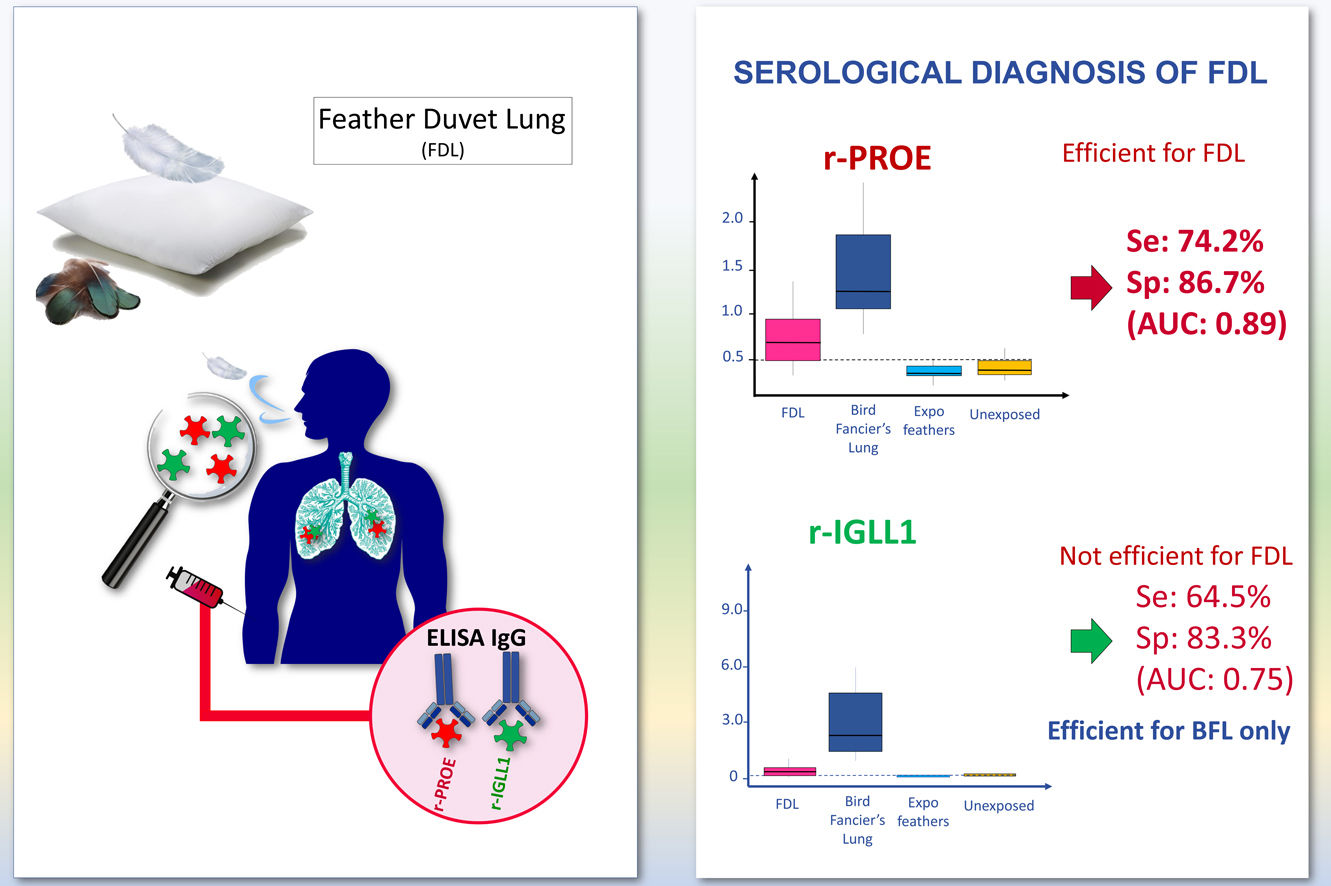
Feather duvet lung (FDL) is an underestimated form of acute and chronic hypersensitivity pneumonitis. Serological tests for FDL need to be validated. We investigated the ability of recombinant pigeon Proproteinase E (r-PROE) and Immunoglobulin-lambda-like-polypeptide-1 (r-IGLL1) proteins to support the serological diagnosis of FDL, and propose them as a serological tool for clinicians to differentiate cases from FDL and Bird fancier's lung (BFL).
MethodsSpecific IgG antibodies against r-PROE and r-IGLL1, analyzed with ELISA, were measured in patients diagnosed with FDL (n=31), BFL (n=15) controls exposed (n=15) and unexposed to feathers (n=15).
ResultsThe sensitivity and specificity of the r-PROE ELISA for the serological diagnosis of FDL cases versus exposed and unexposed controls were 74.2% and 86.7% respectively, with an index threshold of 0.5 (AUC: 0.89). In addition, this serological test was effective to support the serological diagnosis of FDL and BFL cases with significantly different thresholds. The r-IGLL1 ELISA was only effective for the serological diagnosis of BFL. Also, these two serological tests were useful for the diagnosis of both chronic and acute forms.
ConclusionsThe new diagnostic test for FDL using r-PROE protein should help to detect overt and hidden cases of FDL. The combination of both test will help the clinician in distinguish between the etiology of birds or feathers duvet.
Hypersensitivity pneumonitis (HP) is an inflammatory and/or fibrotic diffuse parenchymal lung disease, arising in susceptible individuals after repeated and prolonged inhalation to specific antigens.1 In general, its diagnosis is based on a combination of clinical, radiological, histological, and biological features.1–3
One of the most common forms of HP is bird-fancier's lung (BFL), caused by exposure to bird droppings, feathers and bloom (a waxy powder that coats the feathers).4 However, exposure to feather/down proteins hidden in commonly used objects is involved in another group of avian HP called feather duvet lung (FDL). Feather pillows and down duvets are the main antigenic sources due to their proximity to the respiratory tract and long duration of daily exposure. Exposure to feathers from bedding has been assessed at 30% of the population from data collected at the Vall d’Hebron5 and Besancon Hospital (personal data). In the center of Catalonia, the prevalence of FDL for a period of 10 years has been estimated at 6.2/100,000 users of feather bedding compared to a prevalence of 54.6/100,000 among bird breeders.5 FDL is characterized as an under-recognized and consequently underestimated form of HP in several studies.6–8 In some cases, if not diagnosed early enough, the disease can progress to irreversible pulmonary fibrosis, leading to permanent damage and the premature death of the patient.9
A recent modified Delphi survey on chronic HP shows that exposure to a causative antigen is the most important clinical variable supporting a confident diagnosis.2 Although the use of serological tests is not universally accepted, they can be used to demonstrate antigenic exposure by looking for circulating precipitins or IgG antibodies.2,10,11
The antigens routinely used in the diagnosis of FDL are purified mixtures of goose or duck feather whose performance varies from one batch to another. The identity of the antigens involved in FDL is currently unknown. Recent studies have characterized two pigeon proteins called immunoglobulin-lambda-like-polypeptide-1 (IGLL1) and proproteinase E (PROE),12 which are useful for the serological diagnosis of BFL.12,13
The IGLL1 protein has been identified in droppings, bloom, and pigeon serum and PROE protein in droppings and bloom.12 A strong correlation has been shown between the IgG antibody response of patients sensitized to pigeon, duck and goose antigens.10 Therefore, we hypothesize that amino-acid sequences close to those found in IGLL1 and PROE are part of proteins from the feathers (goose, duck) used for the manufacture of pillows and duvets.
This study aimed (i) to assess the performance characteristic of r-IGLL1 and r-PROE (ii) to support the serological diagnosis of FDL, compare the level of sensitization of FDL and BFL patients and (iii) to provide an effective and useful tool for clinicians to guide the diagnosis of HP of avian origin.
MethodsStudy designRecruitment of the 31 FDL patients was conducted in the Pneumology Unit at the Hospital Universitari Vall d’Hebron (Barcelona, Catalonia, Spain). The 15 BFL patients and the 30 controls exposed or unexposed to feathers without HP were recruited by the University hospital of Besancon (France). The sera of each patient were collected at the time of diagnosis. All patients recruited have given their informed consent.
Hospital Universitari Vall d’HébronThe criteria used to diagnose HP were those defined by Vasakova et al.11 FDL patients (n=31) were selected from an ongoing prospective study undertaken to evaluate the exposure of environmental factors as potential causative factor/s for new onset interstitial lung disease (ILD). Patients were followed up long-term over the study period from January 2004 to December 2013. The diagnosis was done according to the following main criteria: (i) no exposure to birds or other environmental factors that could induce HP, (ii) Past or present exposure to feathers hidden in the environment. These last two criteria were assessed using a standardized questionnaire,14 (iii) Positive specific inhalation challenge and/or specific IgG in the serum. The following antigenic panels were used to measure serum IgG levels by ELISA: bird feathers, bird serum (goose, pigeon, parrot, parakeet, canary), goose feathers and fungus (Aspergillus, Penicillium, Mucor). These patients were included in a previous study (Ethical Committee number: PR(AG) 165/2016), for more clinical details see reference 5.
Besançon University HospitalAll BFL patients (n=15) were given a diagnosis between September 2010 to January 2016 according to the following criteria15: (i) A well-known bird exposure (detailed questionnaire, positive precipitin serological test), (ii) no past or present exposure to feathers or other environments involved in HP, (iii) symptoms and High-resolution computed tomography (HRCT) compatibles with HP and basal crepitant rales, (iv) Bronchoalveolar lavage (BAL) lymphocytosis and (v) decreased DLCO during exercise. This protocol of recruitment was approved by the local ethics committee (CPP-Est II 15/496). The control groups included:
- -
15 subjects exposed to feathers bedding (“Expo feathers”), but not exposed to birds or other environment at risk of HP at the time of diagnosis.
- -
15 subjects not exposed to feathers bedding (“Unexposed”), birds or any environment involved in HP at the time of diagnosis or in the past.
All analyses were performed on the same day for all patients, i.e. retrospectively to the diagnosis. ELISAs were performed without knowing the clinical status of the sera from the Vall d’Hebron department. Indirect ELISAs using r-PROE and r-IGLL1 were performed in April 2019, in the Parasitology-Mycology department as described by Rouzet et al.12
Briefly, the wells of 96-well plates (PolysorpImmunomodule, Nalge Nunc®, Rochester, UK) were coated by incubation with 100μL of 10μg/mL r-PROE and 5μg/mL r-IGLL1 in phosphate-buffered saline (Sigma–Aldrich®, St Louis, USA) at 4°C overnight. Serum samples were diluted 1/100 in dilution buffer, 100μL deposited in triplicate into the wells, and the plates incubated for 1h at 37°C. Polyclonal rabbit antibodies (anti-IGLL1-PROE) (RD-Biotech®, Besançon, France) were used as a positive control of the test (1.8μg/mL) and as a reference sample (0.4μg/mL). The three optical density values (OD) were blank-corrected and averaged and the standard deviation and variation coefficient calculated. If the coefficient of variation of the triplicate was greater than 20%, the outlier was removed. The ELISAs were carried out twice. An index was calculated as follows: Index=mean OD of the blank-corrected sample replicates/mean OD of the blank-corrected reference sample.
Statistical analysisAll statistical analyses were performed with R 3.5.3 software and a p-value of 0.05 was used to define statistical significance. Receiver-operator characteristics (ROC) analysis (pROC-package) was performed to evaluate the ability of r-PROE and r-IGLL1 in ELISA to discriminate between Patients with FDL (status=1) and controls (exposed and unexposed to feathers, status=0). Therefore, BFL patients have been removed from ROC analysis to avoid overestimation of the performance of these 2 recombinant proteins. The performance of r-PROE and r-IGLL1 was also evaluated to detect the threshold discriminating cases of BFL (status=1) and FDL (status=0). Demographic and clinical data were compared between groups. The chi-square test was used to compared categorical variables. Comparisons of the continuous variables among groups were analyzed by the Mann Whitney U test and the nonparametric Kruskal–Wallis (K) test. Following a significant K test, a multiple comparison post hoc test (KMC) using the kruskalmc function (“pgirmess”package) was performed to make inter-group comparisons.
ResultsClinical featureDemographic and clinical data obtained from patients and controls have been reported in Table 1. The median age was 57 years for the 31 FDL patients (16 women and 15 men), 65 years for the 15 BFL patients (10 women, 5 men), 67.5 years for controls exposed to feathers (8 women and 7 men) and 55 years for unexposed controls (4 women and 11 men). Thirty-three per cent of FDL patients were non-smokers compared to 73% for BFL patients, 67% and 60% respectively for controls exposed and unexposed to feathers. There were no significant differences in median age, sex ratio and smoking habits between the HP and control groups.
Demographic and clinical data of 31 patients with FDL, 15 patients with BFL at the time of diagnosis and controls exposed (n=15) and unexposed to feathers (n=15).
| FDL (n=31) | BFL (n=15) | Expo feathers (n=15) | Unexposed (n=15) | P | |
|---|---|---|---|---|---|
| Age, median (SD), years | 57 (14.5) | 65 (11.6) | 67.5 (18.2) | 55 (12.9) | 0.053 |
| Male sex, n (%) | 15 (48.4) | 5 (33) | 7 (47) | 11 (73) | 0.284 |
| Tobacco use, n (%) | 0.105 | ||||
| Non-smoker | 10 (32.3) | 11 (73.3) | 10 (67) | 9 (60) | |
| Past-smoker | 19 (61.3) | 4 (26.7) | 3 (20) | 4 (27) | |
| Active smoker | 2 (6.5) | 0 | 2 (13) | 2 (13) | |
| Exposure to feather, n (%) | 0.057 | ||||
| Duvet | 18 (58.1) | 0 | 6 (40) | ||
| Pillow | 5 (16.1) | 0 | 9 (60) | ||
| Both | 8 (25.8) | 0 | |||
| Exposure to birds, n (%) | |||||
| Pigeon | 0 | 10 (66.6) | |||
| Parakeet | 0 | 2 (13.3) | |||
| Poultry | 0 | 1 (6.7) | |||
| Several birds | 0 | 3 (20) | |||
| HP classification | 0.209 | ||||
| Chronic HP, n (%) | 20 (64.5) | 6 (40) | |||
| Acute HP, n (%) | 11 (35.5) | 9 (60) | |||
| Symptoms | 0.985 | ||||
| Cought, n (%) | 31 (100) | 12 (80) | |||
| Dyspnea, n (%) | 31 (100) | 14 (93.3) | |||
| Emphysema, n (%) | 0 | 1 (6.7) | |||
| Crackles, n (%) | 18 (45.5) | 10 (66.7) | |||
| Analytical data | |||||
| BAL-Lymphocytes %, median (P25–P75) | 12.5 (7–36.3) | 64 (53.8–67.5) | 0.005* | ||
| Pulmonary function tests | |||||
| FEV1/FVC (%pred), mean (SD) | 83.1 (5.3) | 89.2 (16.4) | 0.398 | ||
| DLCO (% pred), mean (SD) | 50 (13.3) | 49.3 (13.4) | 0.874 | ||
| HRCT | 0.0049* | ||||
| Ground glass opacities (GGO), n (%) | 15 (48.4) | 14 (93.3) | |||
| Reticular, n (%) | 4 (12.9) | 1 (6.6) | |||
| Honeycombing, n (%) | 12 (38.7) | 4 (26.7) | |||
Abbreviations: BAL, Bronchoalveolar lavage; % pred, percent of predicted value; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide (CO).
The antigenic source was a feather duvet for eighteen patients, a feather pillow for five and a combination of both in eight. Of the control group, 6 individuals are exposed to feather duvet and 9 are exposed to pillows. Over sixty-six percent of BFL patients were exposed to pigeons, and only 20% were exposed to more than one species of bird. No FDL patients and controls groups were exposed to birds and no BFL patients were exposed to feathers bedding. Of the patients with FDL, 64.5% had a chronic course of HP against 40% for BFL patients. Cough and dyspnea were the predominant symptoms in the two groups of patients. The distribution of chronic and acute forms as well as the symptoms are not significantly different between the FDL and BFL patient groups.
BAL was performed for 28/31 (90%) patients with FDL and 12/15 (80%) of BFL patients. A BAL lymphocyte count of at least 20% were found in 13/28 (46%) patients FDL and in 12/12 (100%) of BFL patients. The percentage of lymphocyte as well as GGO detection on HRCT scan was significantly higher in BFL patients compared to FDL patients.
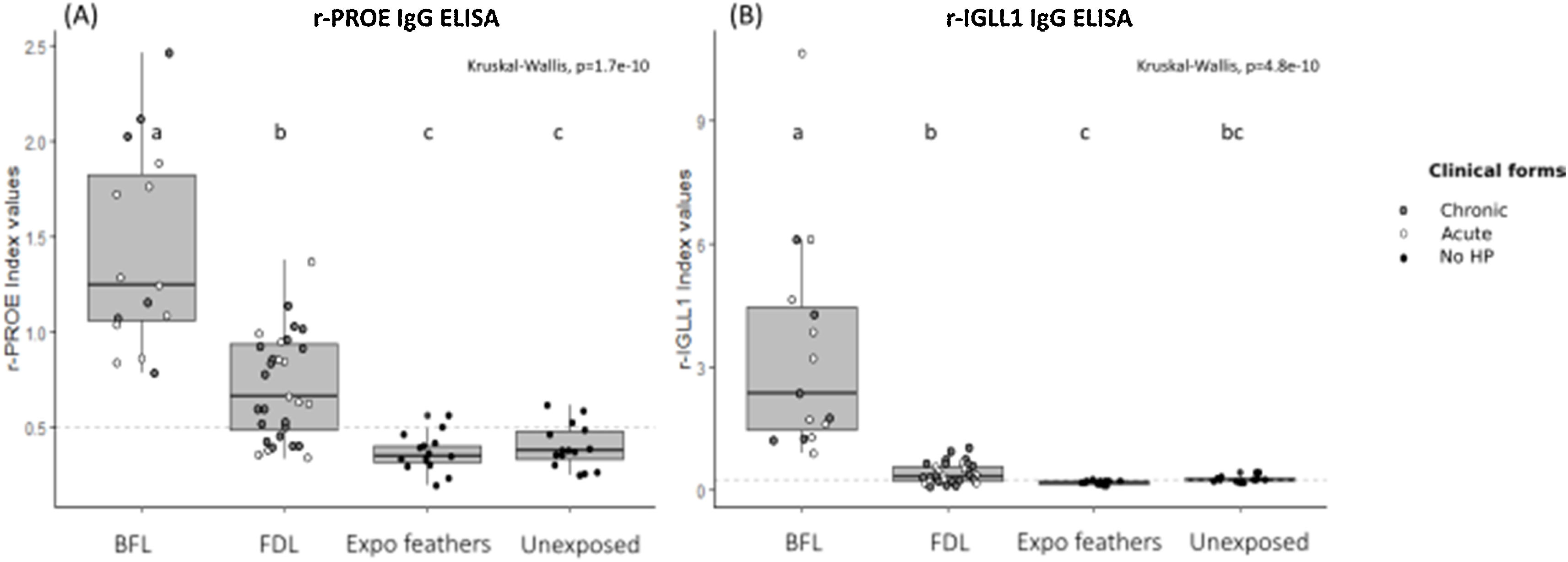
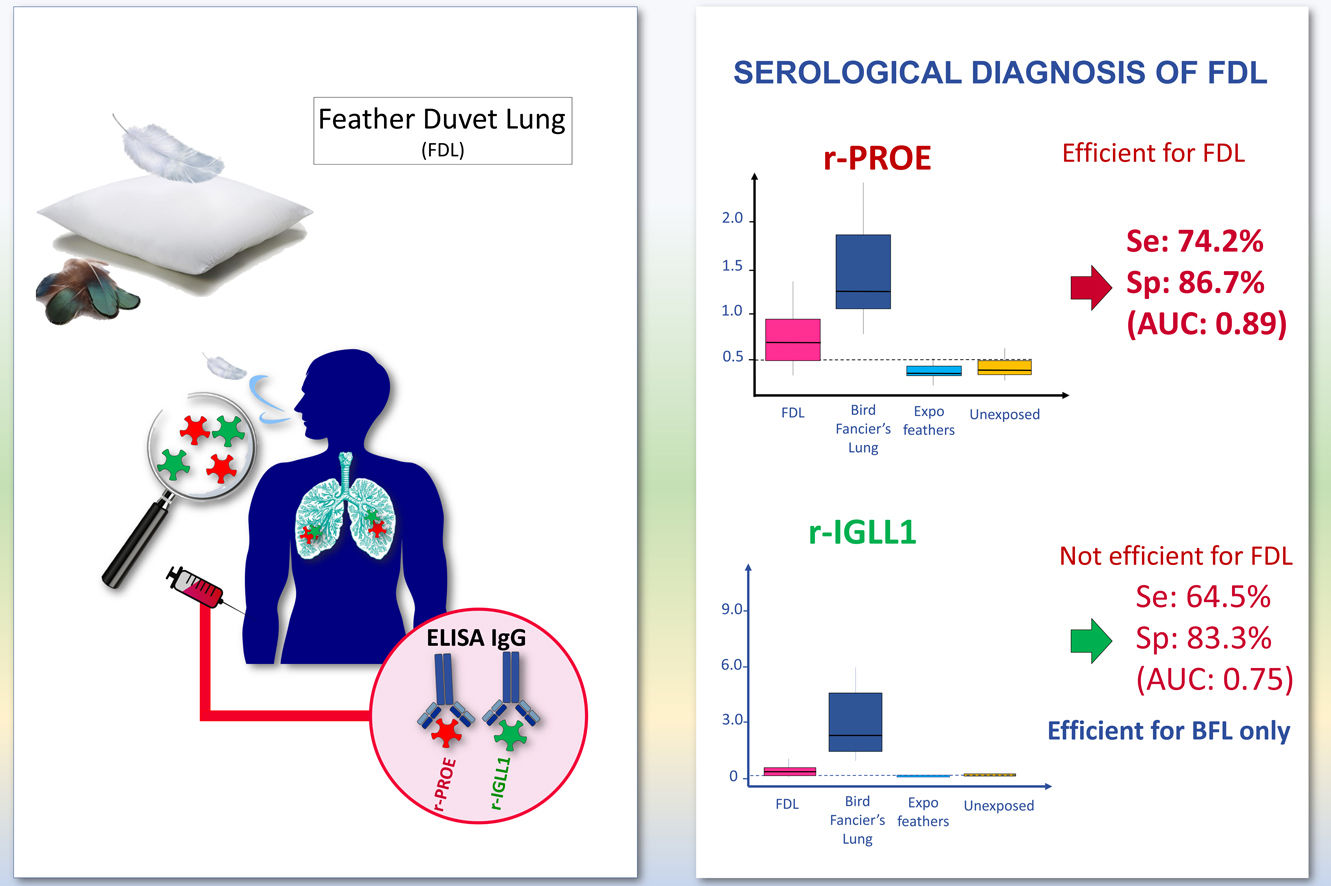
Performance of ELISA serological tests using r-PROE and r-IGLL1The immunization against r-PROE and r-IGLL1 of BFL, FDL patients and control subjects was presented on the boxplots of Fig. 1. As shown in Fig. 1A, BFL patients had significantly higher levels of antibodies to r-PROE than FDL patients as well as controls exposed and unexposed to feathers.
Boxplots of the index values for the r-PROE (A) and r-IGLL1 (B) ELISAs for the different patient groups: FDL (n=31), BFL (n=15), Expo feathers (n=15), Unexposed to feathers (n=15). For FDL and BFL patients, patients with chronic forms are marked by gray circles and those with acute forms by white circles. The horizontal dotted lines represent the threshold determined by the pROC analysis (highest AUC value) for the r-PROE (0.5) and r-IGLL1 (0.3) ELISAs. The letters (a–c) common to several groups indicate no significant difference between the groups (kruskalmc test).
In addition, FDL patients had significantly higher antibody levels than controls exposed and unexposed to feathers. On the other hand, as shown in Fig. 1B, the level of antibodies to r-IGLL1 was significantly higher in BFL patients compared to FDL patients, controls exposed and unexposed to feathers. However, no-significant difference using kruskalmc between FDL patients and controls unexposed to feathers were found. Consequently, r-IGLL1 was contributive to the diagnosis for the serological diagnosis of patients with BFL but ineffective to support FDL diagnosis.
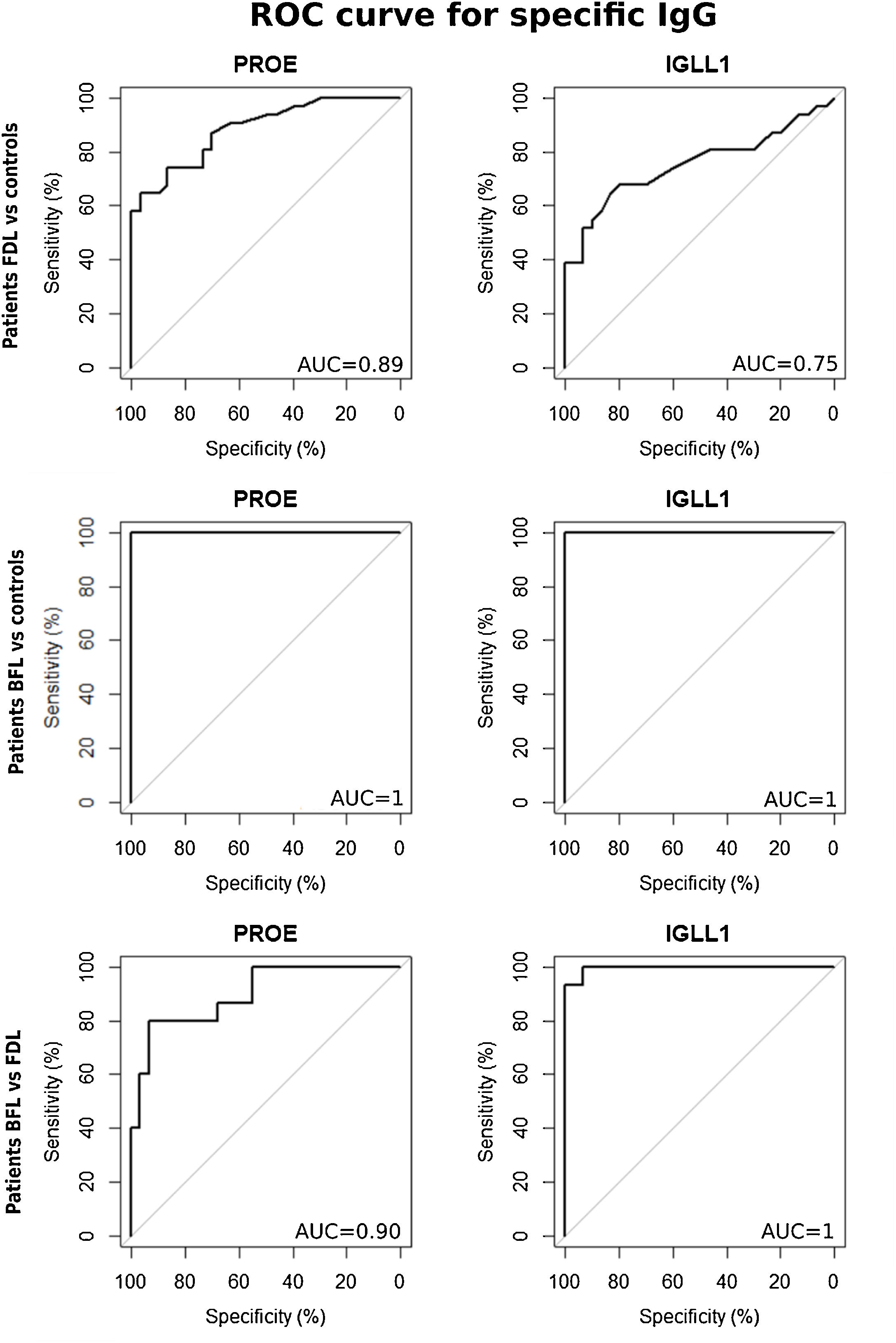
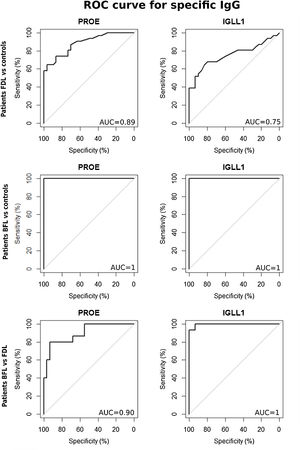
Based on ROC analyses between FDL patients and the controls groups, the area under the curve (AUC) of sIgG was 0.89 using r-PROE antigens, but was 0.75 using r-IGLL1 (Fig. 2). As shown in Table 2, the optimal threshold index of sIgG for r-PROE was 0.505, the sensitivity was 74.2%, and the specificity 86.7%. The r-PROE ELISA test showed a positive result for 85% (PPV) of the FDL patients and negative for 76% of the controls (NPV).
ROC curves for the titers of serum IgG against r-PROE and r-IGLL1 to compare the index values between patients with FDL and controls and between patients with BFL and controls. In the first case, BFL patients were removed to avoid overestimation of the performance of these recombinant proteins. ROC analyses were also performed between FDL and BFL cases by removing controls, in order to highlight a threshold discriminating the cases of FDL and BFL.
Diagnostic accuracy of serum IgG antibody against r-PROE (Proproteinase E) and r-IGLL1 (Immunoglobulin lambda-like polypeptide-1) for FDL and BFL patients.
| Recombinant proteins | AUC [95% CI] | Threshold index value | Sensitivity (%) [95%CI] | Specificity (%) [95%CI] | p-Value (K-test) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|---|
| FDL patients vs. controls (excluding BFL) | r-PROE | 0.89 [0.80–0.95] | 0.505 | 74.2 [58.1–90.3] | 86.7 [73.3–96.7] | 1.449e–06 | 85.19 | 76.47 |
| r-IGLL1 | 0.75 [0.62–0.87] | 0.265 | 64.5 [48.4–80.6] | 83.3 [70–96.6] | 0.000231 | 77.78 | 70.59 | |
| BFL patients vs. controls (excluding FDL) | r-PROE | 1.0 [1.0–1.0] | 0.695 | 100 [100–100] | 100 [100–100] | 3.681e–07 | 100 | 100 |
| r-IGLL1 | 1.0 [1.0–1.0] | 0.665 | 100 [100–100] | 100 [100–100] | 5.579e–08 | 100 | 100 | |
| BFL patients vs FDL patients | r-PROE | 0.90 [0.80–0.98] | 1.035 | 80.0 [60.0–100] | 93.5 [83.9–100] | 1.377e–05 | 85.71 | 90.32 |
| r-IGLL1 | 1.0 [0.98–1.0] | 1.130 | 93.3 [80.0–100] | 100 [100–100] | 6.591e–08 | 100 | 96.77 |
The threshold index value was derived from the ROC analysis. PPV, positive predictive value; NPV, negative predictive value.
This ELISA test was useful to support the serological diagnosis of chronic and acute forms of FDL. Based on ROC analyses between chronic and acute FDL patients and controls, the AUC of sIgG was greater than 0.85 (Figure A1). For an optimal threshold index between 0.5 and 0.61, the minimum sensitivity of this test was 72.7% and specificity 86.7% (Table A2).
Differentiating between the chronic and acute forms of FDL patients did not improve the results obtained for the r-IGLL1 ELISA test. The recombinant proteins PROE and IGLL1 were both effective in supporting the serological diagnosis of BFL patients (Fig. 2, Table 2).
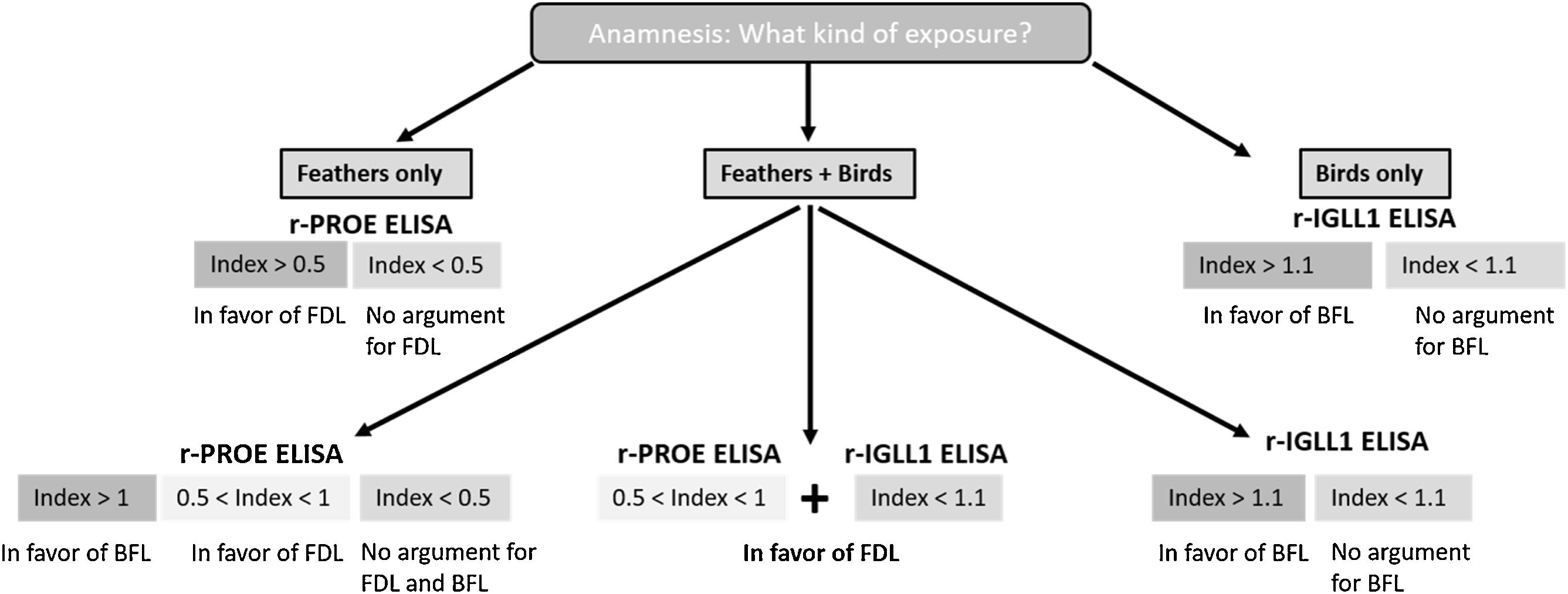
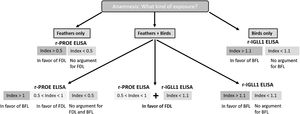
In the case of exposure to both feathers bedding and birds, analyses of the ROC curve showed a significant differential threshold between FDL and BFL cases. If the r-PROE ELISA test index value was between 0.5 and 1, the interpretation was in favor of FDL, while the “bird” etiology was preferred for an index value greater than 1 (Table 2). In addition, if the bird is the cause of the disease, the index value of the r-IGLL1-ELISA test will be greater than 1.1. Based on these results, we have proposed a key choice to support the diagnosis of HP of avian origin according to the patient's exposure, in Fig. 3.
Discussion and conclusionsThe development of tools to improve the diagnosis of HP is a challenge and at the heart of current concerns, as HP experts have highlighted the need for a diagnostic test to measure serum IgG levels with a well-accepted threshold.9 In the present study, we showed that an ELISA test using r-PROE allowed effective discrimination between 31 FDL patients and 30 controls. The r-IGLL1 ELISA test was only useful for the serological diagnosis of the 15 BFL patients. These results will serve as a guide for the clinicians in the choice and interpretation of serological tests to be performed according to the type of avian exposure of patients.
The detection of antigen-specific IgG antibodies is useful to support the diagnosis of HP, as it allows identification of the causal antigen.9,11 In our experience, serological analyses were mainly used to rule out the diagnosis of HP in favor of other respiratory pathologies,16 but also to identify the etiologic agent involved in HP. For serological analyses, several methods to determine precipitins (Ouchterlony double diffusion and Immunoelectrophoresis)17 or specific IgG antibodies (ELISA and ImmunoCAP®, Uppsala, Sweden) have been used in analytical laboratories.18 ELISA was described as being more sensitive than precipitin assays in detecting antibodies to pigeon droppings for BFL serodiagnosis.12,17 Currently, the antigens used for the serological diagnosis of FDL are purified (commercial or non-commercial) from goose feathers, duck feathers, a mixture of both, or from those of other bird species (pigeon, parakeet).19,20 Several studies have shown significantly high antibody levels in patients using feathers duvet and pillow antigens than controls.19–21 Comparison of the data obtained is difficult due to the different techniques used but also to the lack of standardization of antigen production.10
IgG antibodies against r-IGLL1 and r-PROE have been identified as biomarkers of BFL and have been found in droppings, bloom, and pigeon serum using an optimal immunoproteomic approach.12,13 Several studies have suggested the presence of cross-reactive antigenic reactions between different bird species or different avian matrices, especially for pigeons.13,22–24 Recently, significant correlations have been found in serological analyses between pigeon, duck, and goose antigens.10 Based on the BLASTp alignment, our results showed that the amino-acid sequence of duck IGLL has higher identity with goose IGLL (ID: 81%) than pigeon IGLL1 (ID: 65%) (Supplemental data). On the contrary, the amino-acid sequence of duck PROE has higher sequence identity with pigeon PROE (81%) than with the orthologous protein in geese (73%). The conservation of the amino-acid sequences of these proteins is a contributing factor to the antigenic cross-reaction observed in serological analyses.13,23 In the present study, we evaluated the performance of an ELISA using the pigeon r-IGLL1 and r-PROE proteins to support the serological diagnosis of FDL cases.
We found the ELISA test using r-IGLL1 to be useful to support the diagnosis of BFL patients but not for FDL cases. Conversely, we found significantly higher levels of circulating IgG antibodies against r-PROE in BFL and FDL patients than controls. Indeed, r-PROE was the most effective antigen for discriminating FDL patients from controls exposed and unexposed to feathers. The characteristic performance of the r-PROE ELISA test using an optimal threshold index value of 0.5 was as follow: sensitivity of 74.2%, specificity of 86.7% and AUC of 0.89.
The antibodies of patients directed against r-IGLL1 and r-PROE are respectively two and seven times significantly higher in BFL patients compared to FDL patients. Although patients were exposed for long periods to their pillows, but with little agitation, the amount of inhalable antigenic protein was probably lower than that inside an aviary (66.6% of our BFL patients were pigeon breeders). Indeed, in such a location, antigens come from droppings, bloom, and feathers and are frequently suspended by the birds or the breeder during cleaning. Such differences in exposure intensity may explain the higher level of antibodies in BFL patients compared to the FDL observed in our study.
Since the intensity of sensitization is significantly different between BFL and FDL patients against r-PROE in ELISA (r-IGLL1 specific for BFL patients), we proposed a useful key for cases of patients exposed to both feathers and birds. Although r-PROE and r-IGLL1 were both effective for serodiagnosis of BFL cases, to simplify procedures r-IGLL1 could be used only for the diagnosis of patients exposed to birds. Likewise, in the event of a suspected FDL case, r-PROE was the only protein to be used for the ELISA test.
In case of multi-exposure (feathers bedding+birds), an index value for r-PROE between 0.5 and 1 is in favor of the diagnosis of FDL, and above 1, in favor of BFL case. Finally, as there is no effective treatment to reverse the lung damage caused by the disease, early identification of the antigenic source is necessary. The diagnosis of FDL should be based on a proactive approach to find the antigen source to remove it from the patient's environment.16
The main limitations of this study are related to retrospective patient recruitment. Indeed, the sera were stored at −80°C for different periods of time, which may alter the quality of the samples.
In conclusion, this preliminary study provides interesting results and offers a new test for the serological diagnosis of FDL cases. In addition, it proposes a new strategy to identify the etiology involved in case of exposure to both bed feathers and birds. The antigens r-PROE and r-IGLL1 could be used respectively for the serological diagnosis of patients exposed strictly to feathers bedding or birds and presenting with respiratory symptoms.
These two ELISA tests allow the diagnosis of both chronic and acute forms of FDL and BFL cases. The ELISA test based on the use of r-PROE showed a sensitivity of 74.2% and a specificity of 86.7% for an AUC of 0.89 for FDL patients. The use of recombinant proteins guarantees highly standardized production and optimal inter-batch reproducibility and reduces the time taken to report results to patients to 3 days. This time saving allows the implementation of an early avoidance strategy favorable to the patient's state of health.
Conflict of interestNone.
This work was funded by the API3A call for projects from the Besançon University Hospital located in the city of Besançon in France (N° 2015-A01803-46) [API3A (Appel à Projet Interne “3 axes”), HYPERSENS]. The authors warmly thank the PEA2t platform (Chrono-environment, University of Bourgogne Franche-Comté, France), which manages and maintains the analytical equipment used in this study. This study was approved by the local ethics committee in Besancon (CPP-Est II 15/496) and by the team institution Ethics Committee in Barcelona (PR (AG) 165/2016). The patients who are included in the study have given their informed consent.