Surgical lung biopsy (SLB) is recommended for patients with newly detected interstitial lung disease (ILD) when diagnosis remains unclear after clinical, radiological, and sometimes bronchoscopic evaluation.1 However, postoperative complications, including pneumothorax, are particularly concerning in patients with pleuroparenchymal fibroelastosis (PPFE), leading some clinicians to avoid SLB for suspected PPFE.2,3 Patients with PPFE often experience upper lobe volume loss4 and decreased lung compliance due to thoracic cage flattening4–7 and subpleural fibrosis.4,6 SLB can further reduce lung volume, and the stiffened lung requires high pressure to expand, potentially intensifying intrathoracic negative pressure, resulting in residual air space. To our knowledge, no publications have reported detailed results of SLB in PPFE. Therefore, we aimed to investigate the outcomes of SLB in PPFE compared to other ILDs.
This study was approved by the Institutional Review Board of Hokkaido University Hospital (approval number: 024-0050). We retrospectively reviewed data of 39 consecutive patients who underwent SLB for suspected ILD at our department between January 2013 and March 2023. SLB was indicated for patients with suspected ILD, particularly those requiring a definitive diagnosis based on surgical specimens. Biopsy sites were determined following preoperative multidisciplinary discussion (MDD). All patients underwent video-assisted thoracic surgery with partial lung resection using a surgical stapler. The final diagnosis was established through MDD. Of the 39 patients, 10 and 29 patients were included in the PPFE and non-PPFE groups, respectively. In the non-PPFE group, 6 patients were diagnosed with idiopathic pulmonary fibrosis (IPF), 13 with idiopathic nonspecific interstitial pneumonia (NSIP), 2 with combined pulmonary fibrosis and emphysema including one with pulmonary arterial hypertension, 1 with desquamative interstitial pneumonia (IP), 1 with unclassifiable IP, 4 with connective tissue disease-related IP, 1 with hypersensitivity pneumonia, 1 with lymphangioleiomyomatosis.
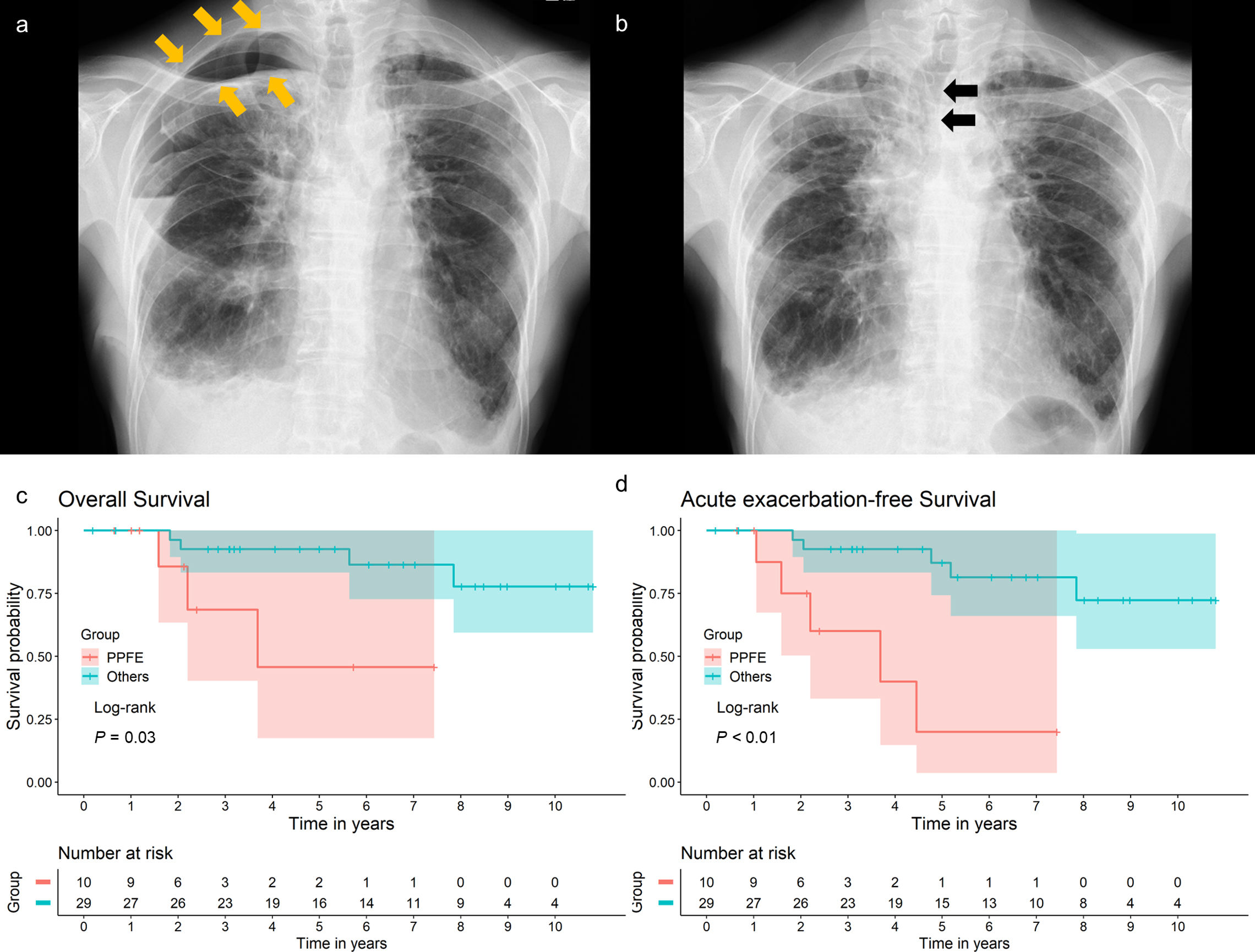
The modified Medical Research Council dyspnea scale was assessed following the established guidelines.8 The resected lung volume was calculated. If multiple lung sections were resected, their sum was considered as the volume of the resected lung. The diagnosis of acute exacerbation (AE) was made based on established criteria.9 Postoperative residual air space was identified on chest radiograph as in routine practice, after confirming the absence of air leaks and removal of the chest tube (Fig. 1a). All statistical analyses were performed using R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria). All analyses were two-tailed, and statistical significance was set at P<0.05.
(a) Chest radiograph of a patient with PPFE at discharge, showing residual air space around the lung apex (arrows). (b) Chest radiograph of the same patient taken 1 month after surgical lung biopsy, showing a mediastinal shift toward the operative side (arrows). (c) Kaplan–Meier survival curves for the overall survival of patients grouped by diagnosis. P=0.03 by the log-rank test. (d) Kaplan–Meier survival curves for the acute exacerbation-free survival of patients grouped by diagnosis. P<0.01 by the log-rank test. Overall survival was defined as the number of days from surgical lung biopsy to death due to any cause or the last follow-up. Acute exacerbation-free survival was defined as the number of days from surgical lung biopsy to the diagnosis of acute exacerbation, death due to any cause, or last follow-up. The final follow-up was conducted in March 2024 to evaluate acute exacerbation, pneumothorax, and survival outcomes.
Abbreviation: PPFE, pleuroparenchymal fibroelastosis.
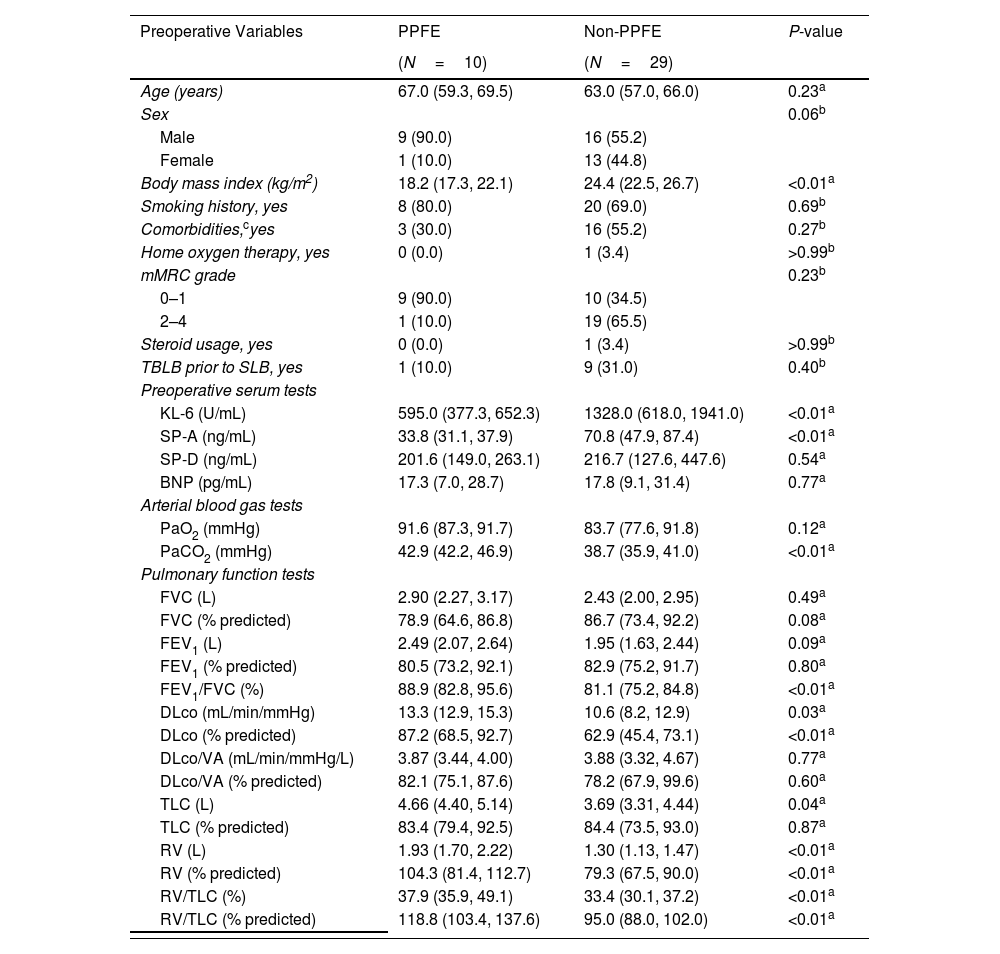
The baseline characteristics of the patients and perioperative outcomes are summarized in Table 1. These characteristics align with previously reported features of patients with PPFE.6,7,10 No cases of prolonged air leak (≥7 days), mortality within 90 days, or AE within 90 days post-SLB were observed in the PPFE or non-PPFE groups. However, the 30-day complication rate was higher in the PPFE group than in the non-PPFE group (20.0% vs. 6.9%, P=0.27). Residual air space at discharge was significantly more frequent in the PPFE group than in the non-PPFE group (60.0% vs. 10.3%, P<0.01). All residual airspaces were classified as small based on the proposed guideline.11 All residual air spaces resolved within 3 months post-SLB. Ipsilateral pneumothorax occurred significantly more often during long-term follow-up in the PPFE group than in the non-PPFE group (30.0% vs. 0.0%, P=0.01). None of the three patients who developed ipsilateral pneumothorax required drainage; one of them had residual air space at 3 months post-SLB.
Preoperative Variables and Perioperative Outcomes in Patients With PPFE and Non-PPFE.
| Preoperative Variables | PPFE | Non-PPFE | P-value |
|---|---|---|---|
| (N=10) | (N=29) | ||
| Age (years) | 67.0 (59.3, 69.5) | 63.0 (57.0, 66.0) | 0.23a |
| Sex | 0.06b | ||
| Male | 9 (90.0) | 16 (55.2) | |
| Female | 1 (10.0) | 13 (44.8) | |
| Body mass index (kg/m2) | 18.2 (17.3, 22.1) | 24.4 (22.5, 26.7) | <0.01a |
| Smoking history, yes | 8 (80.0) | 20 (69.0) | 0.69b |
| Comorbidities,cyes | 3 (30.0) | 16 (55.2) | 0.27b |
| Home oxygen therapy, yes | 0 (0.0) | 1 (3.4) | >0.99b |
| mMRC grade | 0.23b | ||
| 0–1 | 9 (90.0) | 10 (34.5) | |
| 2–4 | 1 (10.0) | 19 (65.5) | |
| Steroid usage, yes | 0 (0.0) | 1 (3.4) | >0.99b |
| TBLB prior to SLB, yes | 1 (10.0) | 9 (31.0) | 0.40b |
| Preoperative serum tests | |||
| KL-6 (U/mL) | 595.0 (377.3, 652.3) | 1328.0 (618.0, 1941.0) | <0.01a |
| SP-A (ng/mL) | 33.8 (31.1, 37.9) | 70.8 (47.9, 87.4) | <0.01a |
| SP-D (ng/mL) | 201.6 (149.0, 263.1) | 216.7 (127.6, 447.6) | 0.54a |
| BNP (pg/mL) | 17.3 (7.0, 28.7) | 17.8 (9.1, 31.4) | 0.77a |
| Arterial blood gas tests | |||
| PaO2 (mmHg) | 91.6 (87.3, 91.7) | 83.7 (77.6, 91.8) | 0.12a |
| PaCO2 (mmHg) | 42.9 (42.2, 46.9) | 38.7 (35.9, 41.0) | <0.01a |
| Pulmonary function tests | |||
| FVC (L) | 2.90 (2.27, 3.17) | 2.43 (2.00, 2.95) | 0.49a |
| FVC (% predicted) | 78.9 (64.6, 86.8) | 86.7 (73.4, 92.2) | 0.08a |
| FEV1 (L) | 2.49 (2.07, 2.64) | 1.95 (1.63, 2.44) | 0.09a |
| FEV1 (% predicted) | 80.5 (73.2, 92.1) | 82.9 (75.2, 91.7) | 0.80a |
| FEV1/FVC (%) | 88.9 (82.8, 95.6) | 81.1 (75.2, 84.8) | <0.01a |
| DLco (mL/min/mmHg) | 13.3 (12.9, 15.3) | 10.6 (8.2, 12.9) | 0.03a |
| DLco (% predicted) | 87.2 (68.5, 92.7) | 62.9 (45.4, 73.1) | <0.01a |
| DLco/VA (mL/min/mmHg/L) | 3.87 (3.44, 4.00) | 3.88 (3.32, 4.67) | 0.77a |
| DLco/VA (% predicted) | 82.1 (75.1, 87.6) | 78.2 (67.9, 99.6) | 0.60a |
| TLC (L) | 4.66 (4.40, 5.14) | 3.69 (3.31, 4.44) | 0.04a |
| TLC (% predicted) | 83.4 (79.4, 92.5) | 84.4 (73.5, 93.0) | 0.87a |
| RV (L) | 1.93 (1.70, 2.22) | 1.30 (1.13, 1.47) | <0.01a |
| RV (% predicted) | 104.3 (81.4, 112.7) | 79.3 (67.5, 90.0) | <0.01a |
| RV/TLC (%) | 37.9 (35.9, 49.1) | 33.4 (30.1, 37.2) | <0.01a |
| RV/TLC (% predicted) | 118.8 (103.4, 137.6) | 95.0 (88.0, 102.0) | <0.01a |
| Outcomes | PPFE | Non-PPFE | P-value |
|---|---|---|---|
| (N=10) | (N=29) | ||
| Number of biopsies | 2.0 (2.0, 2.0) | 2.0 (2.0, 2.0) | 0.25a |
| Biopsy side | 0.03b | ||
| Right | 8 (80.0) | 11 (37.9) | |
| Left | 2 (20.0) | 28 (62.1) | |
| Biopsy site | |||
| Upper lobe/superior segment | 8 (80.0) | 10 (34.5) | 0.03b |
| Middle lobe/lingular segment | 4 (40.0) | 15 (51.7) | 0.72b |
| Lower lobe | 6 (60.0) | 24 (82.8) | 0.20b |
| Resected volume, cm3 | 9.25 (7.15, 45.8) | 19.8 (11.1, 41.6) | 0.59a |
| Operation time, min | 48.5 (42.5, 54.0) | 47.0 (38.0, 61.0) | 0.96a |
| Blood loss, mL | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | |
| Duration of drainage, days | 1.0 (1.0, 1.0) | 1.0 (1.0, 2.0) | 0.61a |
| Postoperative hospital stay, days | 6.0 (5.0, 7.0) | 5.0 (5.0, 6.0) | 0.42a |
| Complications within 30 days, yes | 2 (20.0) | 2 (6.9) | 0.27b |
| Pneumothorax | 1 | 0 | |
| Pleurisy | 1 | 0 | |
| Pneumonia | 0 | 1 | |
| Wound infection | 0 | 1 | |
| Complications after 30–90 days, yes | 1 (10.0) | 0 (0.0) | 0.26b |
| Pneumothorax | 1 | 0 | |
| Residual airspace | |||
| At discharge, yes | 6 (60.0) | 3 (10.3) | <0.01b |
| After 1 month, yes | 2 (20.0) | 0 (0.0) | 0.06b |
| After 3 months, yes | 1 (10.0) | 0 (0.0) | 0.26b |
| Events during follow-up | |||
| Acute exacerbation, yes | 2 (20.0) | 2 (6.9) | 0.27b |
| Ipsilateral pneumothorax, yes | 3 (30.0) | 0 (0.0) | 0.01b |
| Mortality, yes | 3 (30.0) | 4 (13.8) | 0.34b |
| Acute exacerbation | 0 | 1 | |
| Disease progression | 1 | 0 | |
| Pneumonia | 2 | 1 | |
| Lung cancer | 0 | 2 | |
Data are presented as numbers (%) or median (interquartile range).
Abbreviations: BNP, brain natriuretic peptide; DLCO, diffusing capacity of carbon monoxide; DLCO/VA, diffusing capacity of carbon monoxide per alveolar volume; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; KL-6, Krebs von den Lungen-6; mMRC, modified Medical Research Council; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; PPFE, pleuroparenchymal fibroelastosis; RV, residual volume; SLB, surgical lung biopsy; SP-A, surfactant protein A; SP-D, surfactant protein A; TLC, total lung capacity; TBLB, transbronchial lung biopsy.
A logistic regression model was used for the univariable and multivariable analyses to identify factors associated with postoperative residual air space. The univariable analysis identified PPFE as a predictor of residual air space post-SLB (PPFE/non-PPFE, odds ratio [OR]=13.0; 95% confidence interval [CI]=2.48–86.6; P<0.01). In the multivariable analysis, even after adjusting for resected lung volume and biopsy site (upper lobe/others), PPFE remained independently associated with residual air space post-SLB (OR=14.9; 95% CI=2.25–149.2; P<0.01).
Kaplan–Meier curves showed that overall survival (OS) and AE-free survival post-SLB were significantly worse in the PPFE group than in the non-PPFE group (P=0.03; Fig. 1c and P<0.01; Fig. 1d, respectively). The median follow-up period was 791 and 2058 days for the PPFE and non-PPFE groups, respectively. The incidence rate of AE was higher in the PPFE group than in the non-PPFE group (20% vs. 6.9%, P=0.27). However, the association between SLB and AE was unclear because the two patients with PPFE developed AE more than 1-year post-SLB (1 and 3.7 years, respectively). Notably, both patients had lower lobe involvement, as observed on CT, which is reported to be associated with poorer survival outcomes.4 Comparison between patients with PPFE and those with IPF and NSIP, which were consistent with the overall results, are shown in the Additional Supplemental Files.
Patients with PPFE frequently develop pneumothorax, with a reported incidence rate of 25–89%,4,12–14 often accompanied by chronic residual air in the chest cavity.12 The high incidence of pneumothorax is believed to be associated with prominent negative intrathoracic pressure and high transpulmonary pressure due to stiff lungs.4 Therefore, SLB is hypothesized to exacerbate negative intrathoracic pressure and transpulmonary pressure in patients with PPFE by further reducing the volume of their stiff lungs. The current study supports this hypothesis, as the incidence rates of residual air space at discharge and ipsilateral pneumothorax during long-term follow-up were significantly higher in the PPFE group than in the non-PPFE group. Lung collapse during SLB and the volume loss in the stiff lung may require time to re-inflate, potentially leading to a residual air space. After healing of a relatively large air space, a mediastinal shift was observed (Fig. 1b), suggesting exacerbated negative intrathoracic pressure. Additionally, OS post-SLB was worse in the PPFE group than in the non-PPFE group, which included six patients with IPF. Exacerbated negative intrathoracic pressure may have been associated with poor outcomes. However, patients with progressive PPFE exhibit poor prognoses, regardless of SLB.13 Further investigation is needed to determine whether SLB contributes to the development of pneumothorax and worsens prognosis in patients with PPFE.
The high prevalence of PPFE in this study may be due to a combination of various factors. Recent development of transbronchial biopsy, including cryobiopsy, allows more accurate diagnosis of other ILDs. However, diagnosis of PPFE with transbronchial biopsy may be often difficult because it requires biopsy from upper lobe. SLB was not performed in patients with typical clinical/radiological findings for a specific diagnosis. Additionally, the prevalence of PPFE is reportedly high in our region.12 Therefore, patients with PPFE are often recommended to undergo SLB compared to those with other ILDs.
The limitation of this study includes its retrospective, single-center study design and limited sample size, necessitating further research to validate the study results. Additionally, other possible confounders might have been present in the multivariable analysis, which were not accounted for.
In conclusion, this study showed that, compared with patients with other ILDs, those with PPFE experienced a higher incidence of residual air space post-SLB, a greater frequency of ipsilateral pneumothorax during long-term follow-up, and worse survival outcomes post-SLB. Despite the limitations, this is the first study to investigate the prognosis post-SLB in patients with PPFE. Alternative diagnostic criteria using clinical and physiological features in patients with PPFE are warranted.
CRediT Authorship Contribution Statement
(I) Conception and design: HS and TN; (II) Administrative support: KO, AFK, HU, MA, and TK; (III) Provision of study materials or patients: HS, TN, KO, AFK, HU, MA, and TK; (IV) Collection and assembly of data: HS; (V) Data analysis and interpretation: HS, TN, HU, KO, and TK; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Ethical ApprovalThis study was approved by the Institutional Review Board of Hokkaido University Hospital (approval number: 024-0050) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The requirement for individual informed consent was waived because of the retrospective nature of the study.
Declaration of Generative AI and AI-assisted Technologies in the Writing Process
No artificial intelligence software or tool were used in this study.
FundingThis research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
We would like to thank Editage (www.editage.com) for English language editing.