Asthma is one of the most common chronic respiratory diseases, which has been growing over the latest two decades. Recent evidence has related the increase in the prevalence and incidence of asthma with the greatest obesity epidemic present in developed countries.1 In the clinical practice, obesity in adults is often defined using exclusively the body mass index (BMI) of 30kg/m2 or higher, although other anthropometric and metabolic measurements could improve evaluation and diagnosis of obesity.2 The epidemiological link between asthma and obesity was first suggested by Camargo et al. in a study involving 85,911 nurses in the United States.3 The study found that the risk of developing late-onset asthma was significantly increased when the BMI was ≥30kg/m2 with an odds ratio of 2.6. Subsequently, several studies were carried out that corroborated the existence of an excess risk of developing asthma in obese subjects compared with non-overweight (or non-obese) subjects, regardless of gender or age.4 Another study reported that increased waist circumference was associated with asthma even among those with a BMI within the normal range.5
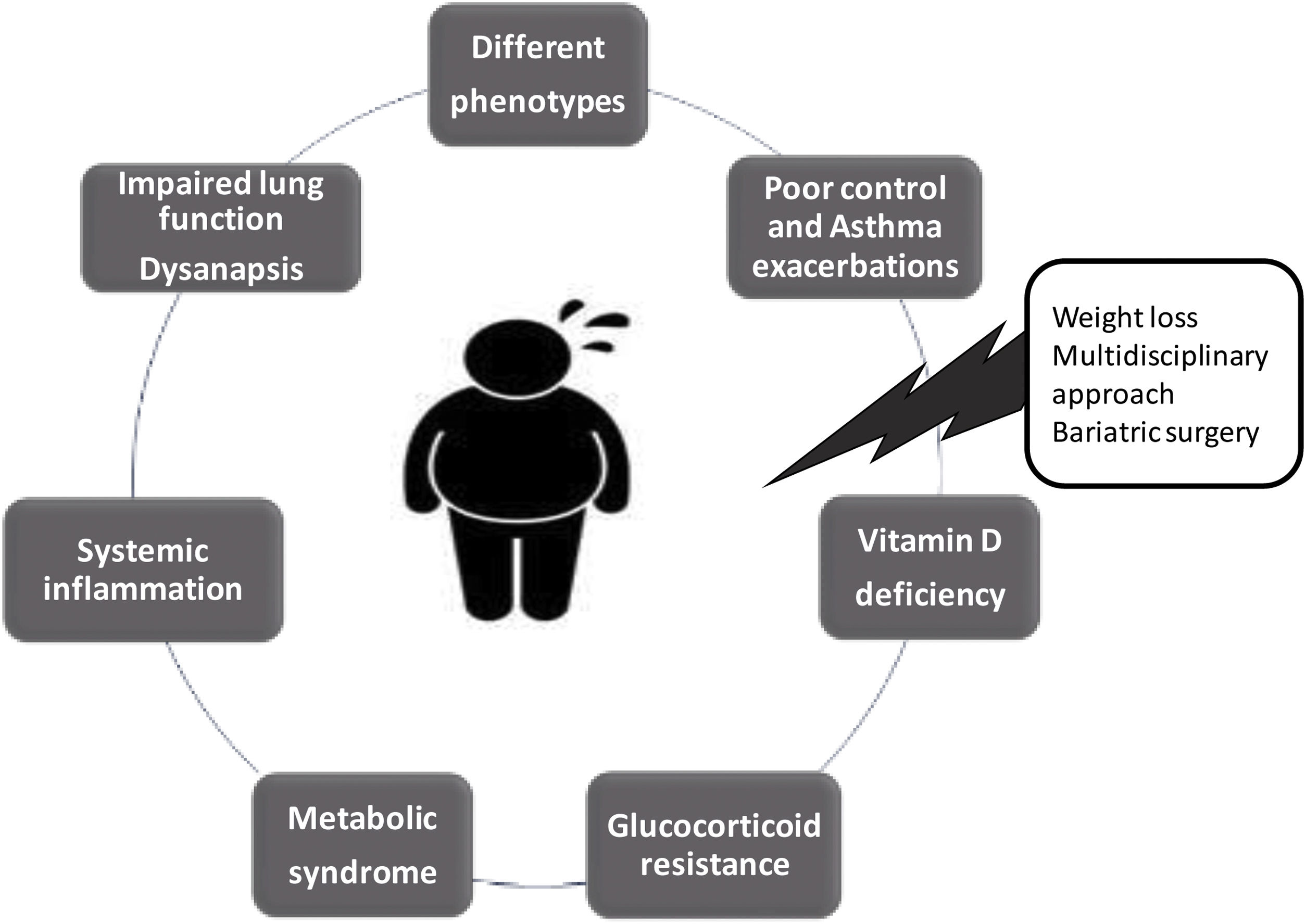
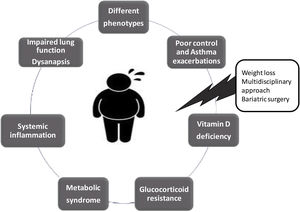
Obesity is one of the major modifiable risk factor of asthma. The association between obesity and asthma, which has been called “Obese Asthma” (OA) has been widely evaluated in the recent years. Several clinical, epidemiological and experimental studies have shown pathways that could link the two processes, including systemic inflammation, lung function alterations, metabolic dysregulation, microbiome changes, and differences in epigenetic/genomic regulation.6 On the one hand, obesity presents low-grade chronic systemic inflammation, which is mainly explained by the adipose tissue, it is metabolically active and releases pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-18, tumour necrosis factor (TNF)-α, Monocyte chemoattractant protein-1 and leptin, an adipocyte-derived hormone that is involved in metabolism and food intake, reproduction, immunometabolism, cancer, among many others.7 Leptin induces allergic inflammatory responses by the proliferation and survival of type 2 innate lymphoid cells (ILC2) and T helper 2 (Th2) cells, and also influences inflammatory responses by inducing the activation of monocytes and both CD4+ and CD8+ T cells,7,8 and the production of pro-inflammatory cytokines such as TNF-α or IL-18, which may affect different respiratory diseases, including asthma.7 Moreover, there is a downregulation of the some of the anti-inflammatory cytokines and adiponectin, an adipokine that counteracts leptin's metabolic effects and inhibits eosinophil recruitment.9 Low levels of adiponectin have been found in patients with obesity and in OA patients. Also, obesity has a different type of effect on lung function in adults (restrictive pattern) and children (obstructive pattern), and recently it has been associated with a phenomenon known as dysanapsis, which results from a disproportionate growth between lung parenchyma size and airway calibre in obese children. Dysanapsis in OA children are associated with increased medication use, more emergency department visits, hospitalizations and systemic corticosteroid burst in comparison with asthma children with normal weight.10,11 Overall, obesity causes a metabolic dysfunction with an imbalance between pro-inflammatory and anti-inflammatory cytokines, with its consequent systemic inflammatory profile and impaired lung function. On the other hand, asthma is typically defined as an inflammatory airway disease, with airway hyperresponsiveness, which frequently presents eosinophilic inflammation whether of allergic or non-allergic origin, and many pro-inflammatory cytokines such as IL-5, IL-13, IL-4, among others, are well known as inflammation type 2 (T2).12 The resulting regulatory effects of the immunomodulatory pathways underlying both diseases have been hypothesized as one of the mechanisms by which obesity increases asthma risk and severity. Ten years ago, Sideleva et al.13 found that adipose tissue inflammation is increased in obese individuals with asthma, compared with obese non-asthmatic controls. Despite the evidence confirming the importance of the OA syndrome is numerous, it is still unknown which biomarkers or components of the metabolic dysfunction present in patients with obesity, are reliable in the mechanism of airway inflammation for developed asthma.14 The complexity of the OA syndrome is exacerbated by the presence of more than one (or many) phenotypes. Two main sub-phenotypes of OA have been described according to age: early-onset and late-onset asthma. Furthermore, Dixon et al.6 propose other phenotypes in OA syndrome: (1) those typically seen in lean individuals, now complicated by obesity; (2) disease newly arising in obese individuals; and (3) a possible separate phenotype characterized by increased response to environmental pollutants. These phenotypes may have various pathological pathways and consequently different management strategies and therapeutic options.
It has been described that OA patients are more likely to have a poor response to glucocorticoids, with reduced odds of achieving asthma control, higher risk of asthma hospitalizations, and lower quality of life compared with asthmatics with a normal BMI. Glucocorticoids have the ability to reduce airway inflammation, airway obstruction, airway hyperresponsiveness, and asthma symptoms. The detrimental effects of obesity on lung function and additive or synergistic effects of obese systemic inflammation on airways inflammation, have been proposed as potential mechanisms to explain glucocorticoid hyporesponsiveness in patients with OA. Moreover, a defective induction of anti-inflammatory genes by glucocorticoids, such as mitogen-activated protein kinase phosphatase-1 (MKP-1), has been studied as another possible mechanism of the reduced response to glucocorticoids seen in OA patients. Besides that, some evidence indicated an association between the risk of glucocorticoid resistance and vitamin D deficiency. Vitamin D is a hormone with pleiotropic effects and numerous regulatory mechanisms beyond bone health. Several observational studies suggest that obesity is associated with vitamin D deficiency. Furthermore, low serum vitamin D levels have been found associated with poor control and asthma exacerbations. There is also in vitro evidence for vitamin D increasing glucocorticoid sensitivity.
Recently, Bantulà et al.15 evaluated patients with obesity and patients with OA before and six months after bariatric surgery, finding a reduced response to dexamethasone in peripheral blood mononuclear cells (PBMCs) of both groups with respect to non-obese asthmatic patients and healthy subjects, this inversely correlated with the adiponectin/leptin ratio and serum vitamin D levels. Bariatric surgery improved corticosteroid responses in patients with obesity and OA patients and normalized the adiponectin/leptin ratio and vitamin D levels. Exposure of PBMCs to vitamin D potentiated the antiproliferative effects of corticosteroids. Dexamethasone and vitamin D induced similar MKP-1 expression in patients with obesity and OA patients. The conclusion was that the efficacy of weight loss to improve symptoms and lung function in OA patients could be, at least in part, due to the recovered anti-inflammatory effects of corticosteroids. Vitamin D deficiency may contribute to corticosteroid hyporesponsiveness in OA.
Finally, bariatric surgery is considered the most effective and sustained long-term treatment of severe obesity. Weight reduction significantly improves systemic and adipose tissue inflammatory activity levels. The literature has showed an improvement in asthma control, medication use, hospitalization rate, and lung function after weight loss in patients undergoing bariatric surgery.16,17 Likewise, lifestyle changes with a weight loss≥10% improve asthma control and reduce exacerbations.
Much research remains to be carried out to understand OA syndrome and its different phenotypes, and the impact of the other therapeutic approaches (Fig. 1). Good clinical practice for respiratory physicians should include assessment of anthropometric and metabolic measurements in all patients with asthma, in order to accurately identify other phenotypes inside of the complexity of OA syndrome.
The authors declare there are no conflicts of interest.