Non-intubated thoracic surgery (NITS) is a burgeoning technique, where patients no longer require intubation to facilitate lung isolation. Thoracic surgery induces a significant systemic and respiratory inflammatory stress response, which is further exacerbated by intubation and mechanical one lung ventilation. The advent of minimally invasive video assisted thoracoscopic surgery (VATS), with its benefits of reduced tissue trauma, shorter recovery times, reduced pain, and improved 5-year mortality1 has led to its widespread use. Along with the reduction in invasiveness of the surgical approach, the anaesthetic approach, with the development of non-intubated thoracic anaesthesia, novel chest wall regional nerve blockades, along with opioid free techniques, has also co-evolved to minimise harm.
It is generally well accepted that gold standard anaesthetic technique for VATS is general anaesthesia combining a hypnotic agent, opioids, and neuromuscular blockade in order to achieve mechanical positive pressure one lung ventilation. However, there are detrimental side effects including atelectasis, ventilator induced lung injury, impaired cardiac function, post operative nausea and vomiting, pneumonia and delirium. In recent years, NITS has emerged as attractive anaesthetic technique based on the concepts learnt from experiences in enhanced recovery after surgery pathways.2 It is widely performed for a variety of procedures including lung cancer surgery (segmentectomies, lobectomies and wedge resections), upper airway surgery, drainage of pleural effusions and bullae excision or surgery for pneumothorax.3 In this editorial we summarise previously published evidence (Table 1) and suggest some of the general principles for developing a safe NITS programme.
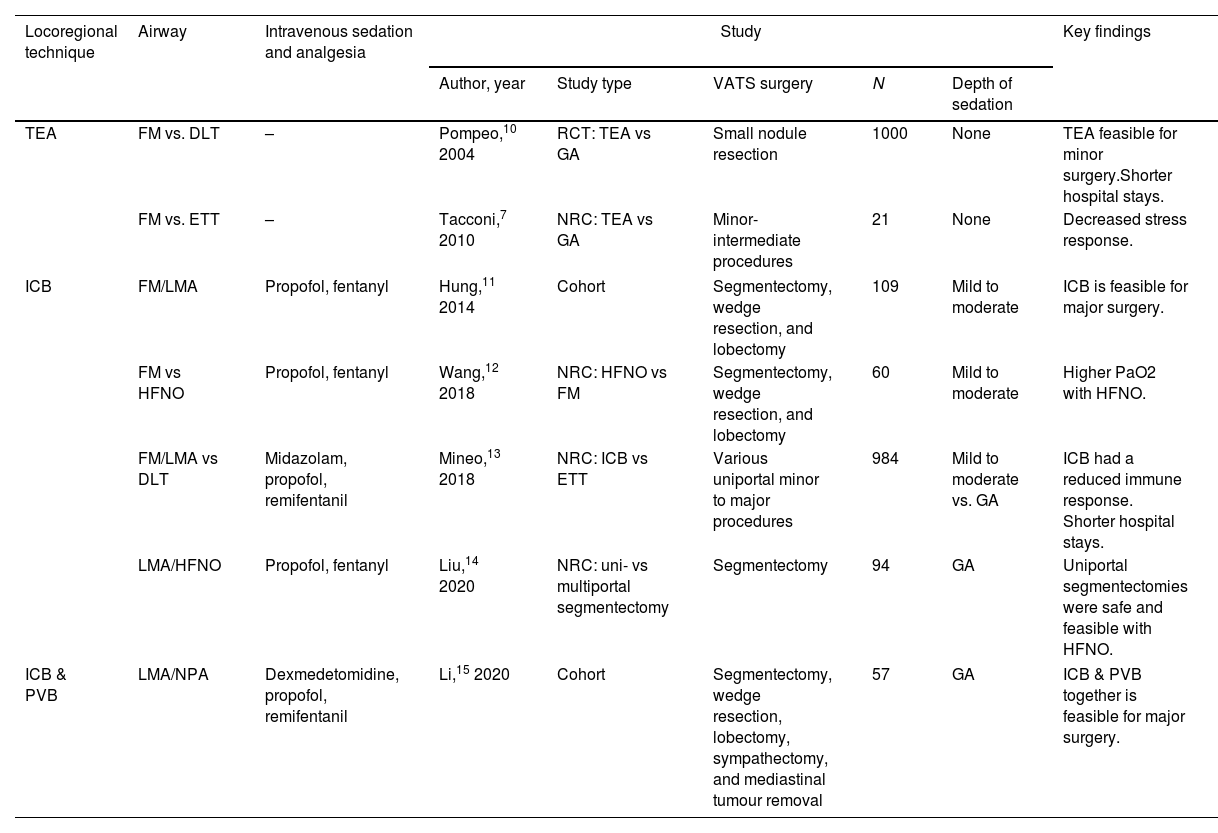
A review of the evidence for NITS.
| Locoregional technique | Airway | Intravenous sedation and analgesia | Study | Key findings | ||||
|---|---|---|---|---|---|---|---|---|
| Author, year | Study type | VATS surgery | N | Depth of sedation | ||||
| TEA | FM vs. DLT | – | Pompeo,10 2004 | RCT: TEA vs GA | Small nodule resection | 1000 | None | TEA feasible for minor surgery.Shorter hospital stays. |
| FM vs. ETT | – | Tacconi,7 2010 | NRC: TEA vs GA | Minor-intermediate procedures | 21 | None | Decreased stress response. | |
| ICB | FM/LMA | Propofol, fentanyl | Hung,11 2014 | Cohort | Segmentectomy, wedge resection, and lobectomy | 109 | Mild to moderate | ICB is feasible for major surgery. |
| FM vs HFNO | Propofol, fentanyl | Wang,12 2018 | NRC: HFNO vs FM | Segmentectomy, wedge resection, and lobectomy | 60 | Mild to moderate | Higher PaO2 with HFNO. | |
| FM/LMA vs DLT | Midazolam, propofol, remifentanil | Mineo,13 2018 | NRC: ICB vs ETT | Various uniportal minor to major procedures | 984 | Mild to moderate vs. GA | ICB had a reduced immune response. Shorter hospital stays. | |
| LMA/HFNO | Propofol, fentanyl | Liu,14 2020 | NRC: uni- vs multiportal segmentectomy | Segmentectomy | 94 | GA | Uniportal segmentectomies were safe and feasible with HFNO. | |
| ICB & PVB | LMA/NPA | Dexmedetomidine, propofol, remifentanil | Li,15 2020 | Cohort | Segmentectomy, wedge resection, lobectomy, sympathectomy, and mediastinal tumour removal | 57 | GA | ICB & PVB together is feasible for major surgery. |
TEA, thoracic epidural anaesthesia; ICB, intercostal nerve block; PVB, paravertebral block; FM, face mask; LMA, laryngeal mask airway; NPA, nasopharyngeal airway; ETT, cuffed endo tracheal tube type; DLT, double lumen tube; BB, bronchial blocker; HFNO, high flow nasal oxygenation; RCT, randomised control study; NRC, non randomised comparison; SV, spontaneous ventilation.
The benefits of spontaneous breathing are obvious for all patients being more notable in patients with reduced lung function4 offering a physiological approach to ventilation. Whilst in the lateral decubitus position, NITS keeps the respiratory muscle tone intact, preventing atelectasis, preserving functional residual capacity, and improving mucocilliary secretion clearance whilst improving ventilation/perfusion matching and oxygenation.
Once chest wall is opened, lung collapse will be induced by an iatrogenic pneumothorax while the non-dependant lung is breathing spontaneously. Moreover, paradoxical movements of the non-dependent lung, as well as the mediastinal shift towards the dependent lung will improve the surgical field. However, in cases of pleural adhesions due to previous surgery or infections such as tuberculosis, lung collapse will be difficult to achieve worsening surgical access to both lung and pleura.
Patient selection is vital, where patients with high body mass index, decompensated heart disease, bleeding disorders and sleep apnoea are often excluded.4 Relative contraindications to NITS include a large hiatus hernia, a known or predicted difficult airway, and pulmonary hypertension where the patient is unlikely to tolerate the cardiovascular instability associated with the hypercapnoea.5
Therefore, the success of NITS requires a three-pronged approach that includes
- •
Locoregional technique
- •
Airway management
- •
Intravenous sedation and analgesia
The use of locoregional anaesthetic techniques, alongside the evolution of VATS has led to the realisation of NITS. Direct or aerosolised vagal blocks have been used to suppress the cough reflex and reduce movements during bronchial dissection. Thoracic epidural anaesthesia has been superseded by paravertebral, erector spinae, serratus anterior and intercostal nerve blocks, because of its favourable side effect profile.6 The use of locoregional techniques was demonstrated to be more advantageous with shorter hospital stay, reduced perioperative fasting times, reduced duration of antibiotic use and reduced stress response.7
The airway management during NITS varies depending on depth of unconsciousness. Whilst in lighter planes of anaesthesia, a facemask or high flow nasal oxygenation (HFNO) could be utilised, a supraglottic airway devices (SAD) such as the laryngeal mask airway, is first option in deeper anaesthesia in order to avoid airway obstruction during spontaneous ventilation. The use of HFNO has been demonstrated to increase the oxygen reserves perioperatively with comparable carbon dioxide concentrations.
The decreased minute volume may result in hypercapnea, which is generally well tolerated. During NITS, an inadequate lung isolation and poor surgical conditions or the decreased efficiency in respiratory mechanics that results in severe hypoxaemia or may trigger the need for conversion to positive pressure ventilation intra-operatively. The need to definitive lung isolation intraoperatively whilst the spontaneously ventilating patient is in the lateral decubitus position, is fraught with difficulty. This conversion often necessitates the increasing the depth of anaesthesia, paralysis, and the introduction of either a bronchial blocker (BB) through the SAD or the insertion of a double lumen tube (DLT). Endobronchial instrumentation for lung isolation is also associated with tracheobronchial rupture (1 in 22,000) with a mortality rate as high as 22%8 particularly with women. Dislodgement of DLTs and BBs may lead to hypoxaemia and require frequent bronchoscopy to confirm their position.
A variety of depths of sedation have been used for NITS where an adequate depth of anaesthesia for thoracic surgery must be balanced with spontaneous ventilation. The choice of sedation includes intravenous agents including midazolam, propofol, opioids as well as sevoflurane for the maintenance of anaesthesia. The use of appropriate monitoring, including end tidal carbon dioxide is vital for ensuring spontaneous ventilation. Intravenous sedative techniques allow for accurate titration of anaesthetic agents with the use of processed electroencephalographic depth of anaesthesia monitoring. The use of intravenous opioids has undesirable side effects, including respiratory suppression, post operative sedation, nausea, and vomiting. The use of opioid free techniques, using magnesium, ketamine, and clonidine have been showed to not only be feasible and desirable but has been associated with lower pain scores and all cause complications.9
The interdisciplinary collaboration between surgeons and anaesthetist is pivotal to the day-to-day success of the technique. The techniques described require appropriate training and daily use with the confidence to be able to respond appropriately to eventual complications. The anaesthetist thus has to be vigilant of the surgical process and act accordingly to provide the adequate anaesthetic conditions for successful surgery. The use of spontaneous ventilation to every day practice, requires appropriate patient selection, training, institutional involvement and good communication between the surgical and anaesthetic teams.
The future of thoracic surgery may involve using robotic assisted minimally invasive thoracic techniques, which is associated with its own anaesthetic considerations specially for performing a capnothorax. These future innovations have to be integrated in the context of thoracic surgery which no longer being synonymous with endotracheal intubation. Anaesthetists will be active players of this new era of non-intubated thoracic surgery where old dogmas on safe provision of one-lung ventilation will be modified. An exciting unchartered route is starting to be uncovered aiming for a less aggressive anaesthesia for evolving thoracic surgery.
FundingAuthors received no external funding.
Conflict of interestsThe authors state that they have no conflict of interests.