Chronic respiratory diseases (CRD) are responsible for more than four million deaths worldwide and have become especially prevalent in developed countries. Although the current therapies help manage daily symptoms and improve patients’ quality of life, there is a major need to prevent exacerbations triggered mainly by respiratory infections. Therefore, CRD patients are a prime target for vaccination against infectious agents. In the present manuscript we review the state of the art of available vaccines specifically indicated in patients with CRDs. In addition to pneumococcus, influenza, pertussis, and SARS-CoV-2 vaccines, recently added immunization options like vaccines and monoclonal antibodies against respiratory syncytial virus, are particularly interesting in CRD patients. As new products reach the market, health authorities must be agile in updating immunization recommendations and in the programming of the vaccination of vulnerable populations such as patients with CRDs. Organizational and educational strategies might prove useful to increase vaccine uptake by CRD patients.
Chronic respiratory diseases (CRDs) are clinical conditions that affect the airways and other lung structures.1 Among the most common CRDs are asthma, chronic obstructive pulmonary disease (COPD), interstitial lung disease, bronchopulmonary cancer, cystic fibrosis, occupational lung diseases, and pulmonary hypertension.1,2 The Global Burden of Disease Study 2019 estimated that CRDs were the third leading cause of death accounting for 4 million deaths, and nearly 455 million prevalent cases worldwide, representing an increase of 29% and 40% in the number of CRD-related deaths and cases since 1990, respectively.3 In 2019, COPD was the primary cause of death from CRDs with over 200 million prevalent cases and 3 million deaths, globally.3 In that year, asthma was the most prevalent CRD affecting more than 260 million individuals,3 and ranked the most frequent CRD among children accounting for 13,000 child deaths.3,4 Interstitial lung disease and pulmonary sarcoidosis were responsible for 3.8 million disability-adjusted life years globally in 2019, with 4.7 million prevalent cases and 24.2 million incident cases.2 Lung cancer remains the leading cause of cancer mortality worldwide, with 1.8 million harvested lives in 2020.5,6
While the full picture of the epidemiology in low and middle-income countries is not fully available, though impressive data have been reported, the burden in the high socioeconomic index countries remains extraordinarily high.3,7 Recognizing the global impact of CRDs, the World Health Organization (WHO) launched a CRD-specific programme to help countries to reduce the toll of morbidity and mortality attributed to CRD, mainly asthma, and COPD.1,8
Current therapies are effective in reducing the chronic symptoms of CRDs,1,9,10 but exacerbations prevention, especially for COPD, can be substantially improved.11 CRDs are mainly exacerbated after exposure to environmental factors.1,9,10 Respiratory viral infections contribute to between 80% and 90% of asthma exacerbations in children and to between 45% and 80% of asthma exacerbations in adults.12 They are also associated with 30% to 80% of COPD exacerbations,12 and can be identified in up to 50% of bronchiectasis exacerbations.13 The ease of viral infection transmission and infection as well as the diversity of the respiratory viruses yield their high involvement in the exacerbations of CRD and related outcomes.12,14,15 The COVID-19 pandemic put into manifestation the impact of viral infections on CRD worsening, where the globe witnessed a decline in the incidence of acute exacerbations of CRDs by around 50%,16 calling for viral infections prevention and control, especially in CRD patients.
Vaccination is the most powerful tool in preventing infection; hence it is a fundamental preventive measure that has been recommended by experts in respiratory diseases and in international clinical guidelines to protect against CRD exacerbations. In some cases, the vaccine will be able not only to protect against clinical disease, but also against infection (i.e. pneumococcal conjugate vaccines), in other cases the vaccine will have limited or no impact on infection acquisition (i.e. COVID-19 vaccines), and for some vaccines the eventual impact on transmission is not known yet (i.e. respiratory syncytial virus [RSV] vaccines). The American Lung Association recommends vaccinating CRD patients against COVID-19 to avoid severe CRD outcomes.17 A review of clinical guidelines on non-communicable diseases with high global disease burden in people 75 years and older found that vaccination against COVID-19, influenza, and pneumococcal diseases has been recommended in international guidelines on the management of CRDs such as COPD and asthma.18 In Spain, the NeumoExperts Prevention (NEP) group developed a practical vaccination guide against community-acquired pneumonia in adults caused by vaccine-preventable diseases, including specific recommendations for adult patients with CRD.19
In the case of COPD patients, the international NIH document Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends influenza vaccine, pneumococcal vaccine, COVID-19; tetanus, diphtheria, pertussis vaccine (Tdap), zoster, and more recently, RSV vaccine.7 National guidelines from the United States, Canada, United Kingdom, and Australia–New Zealand recommend influenza vaccine and six countries recommend pneumococcal vaccine.18
As for asthma, the international NIH document Global Initiative for Asthma (GINA) recommends influenza and COVID-19 vaccines.20 Three national guidelines from the United States and Australia–New Zealand recommend the influenza vaccine, two of those three guidelines promote the pneumococcal vaccine, and collectively, the COVID-19 vaccine is advocated, alongside the previously mentioned vaccines.18
Given the global impact of CRDs on public health and the key role that vaccination plays in preventing virus-induced CRD exacerbation, this work aims to review available and under-development vaccines against the main pathogens associated with CRDs, namely RSV, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), influenza virus, pneumococcus, and Bordetella pertussis.11,21–26
Vaccines for Chronic Respiratory DiseasesRSVRSV contributes substantially to morbidity and mortality burden globally in the very young, elderly, and high-risk groups. Worldwide, in 2019, RSV was associated with 33 million acute lower respiratory tract infection (ALRI) episodes, nearly four million ALRI hospitalizations, more than 26,000 RSV-associated ALRI in-hospital deaths, and 100,000 RSV-attributable overall deaths in children under five, with the greatest impact being observed in newborns and infants younger than six months.27 Recent reports suggest a higher public health burden from RSV than influenza in the child as well as the adult population.28,29 In children younger than one, according to US data collected between 1999 and 2018, the mortality attributed to RSV was fivefold higher than that related to influenza.29 Likewise, in Spain, 9% of hospitalized ALRI cases between 2012 and 2020 were associated with RSV, while only 3% were influenza-related, and in-hospital mortality was higher among RSV than influenza patients.28 In the context of CRDs, several studies concluded that there is an association between severe RSV bronchiolitis and subsequent recurrent wheeze and asthma in later childhood and that previous RSV bronchiolitis is also associated with more severe asthma.27 A study undertaken in the US between 2003 and 2010, reported that there is approximately three to sevenfold higher risk for RSV-related hospitalization in children under five with a prior asthma diagnosis; a risk magnitude surpassing that of influenza.30 Likewise, in a study involving adult patients where pneumonia, COPD, and hypoxemia were frequent, the 20-day all-cause mortality rate was as high as 18% for RSV infections, while it was around 7% for influenza infection.31 The study also found that the risk of death from RSV infection was more than twice that from influenza infection.31 RSV infection was identified in up to 22% of acute COPD exacerbations.32
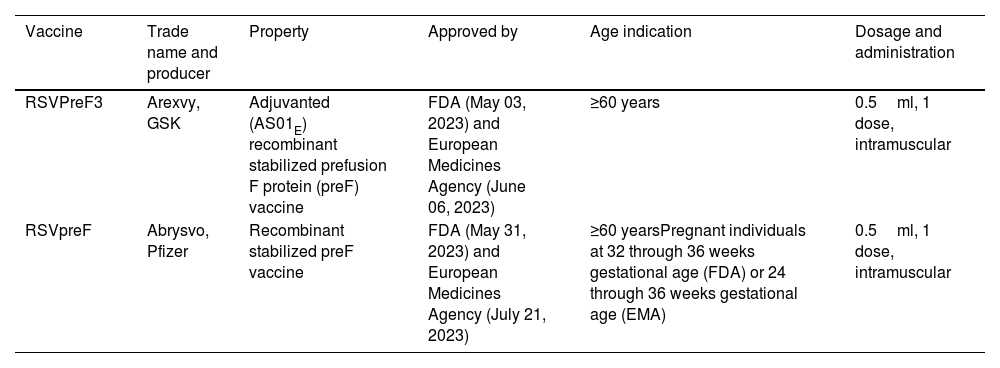
Vaccine development against RSV is a WHO priority.33 Prevention strategies against RSV encompass three target populations: pediatric, maternal, and elderly.34 Currently, two single-dose RSV vaccines have been cleared by the US Food and Drug Agency (FDA) and European Medicines Agency (EMA) for use in elder adults aged 60 and above, RSVPreF3 (Arexvy, GSK) and RSVpreF (Abrysvo, Pfizer) (Table 1).35–40 RSVpreF is also recommended for pregnant individuals.35,39,41 In a phase III clinical trial in adults aged 60 or above (AReSVi 006 Study), the prefusion F protein RSV vaccine, RSVPreF3 (Arexvy, GSK), showed an 83% efficacy for RSV-associated lower respiratory tract disease (LRTD), and 94% for severe RSV-associated LRTD. During the second RSV season post-vaccination, the GSK vaccine efficacy in preventing symptomatic, laboratory-confirmed RSV-LRTD was 56%.40 RSVpreF (Pfizer), is a bivalent RSV-A and RSV-B stabilized prefusion F protein vaccine that, in phase III clinical trial in adults ≥60 years (RENOIR study), showed vaccine efficacy of 67% for RSV-LRTD with at least two clinical signs and 86% for the most severe respiratory disease where at least three symptoms associated with RSV were present.40 The efficacy of RSVpreF was 79% during the partial second RSV season following vaccine administration.40 Both vaccines, RSVPreF3 and RSVpreF, proved safe and well tolerated when simultaneously administered with other adult vaccines such as seasonal influenza and COVID-19 vaccines.42,43 The responses were confirmed in subgroups of patients with specific comorbidities such as cardio-pulmonary, endocrine and metabolic comorbidities, where vaccine efficacy was similarly obtained. Based on this specific evidence, GOLD 2024 included the recommendation for RSV vaccination in COPD subjects with a level A of evidence, at variance with other vaccinations that are reported with a B or lower level of evidence.44,45
Summary of characteristics of RSV vaccines licenced by US Food and Drug Administration and European Medicine Agency for use in adults (as of Septemeber 2023).
| Vaccine | Trade name and producer | Property | Approved by | Age indication | Dosage and administration |
|---|---|---|---|---|---|
| RSVPreF3 | Arexvy, GSK | Adjuvanted (AS01E) recombinant stabilized prefusion F protein (preF) vaccine | FDA (May 03, 2023) and European Medicines Agency (June 06, 2023) | ≥60 years | 0.5ml, 1 dose, intramuscular |
| RSVpreF | Abrysvo, Pfizer | Recombinant stabilized preF vaccine | FDA (May 31, 2023) and European Medicines Agency (July 21, 2023) | ≥60 yearsPregnant individuals at 32 through 36 weeks gestational age (FDA) or 24 through 36 weeks gestational age (EMA) | 0.5ml, 1 dose, intramuscular |
Various vaccines of different natures against RSV in older adults are in the research pipeline. Recombinant vector-based vaccines aiming at eliciting both humoral and cellular immune responses against RSV are under clinical investigation,46 with two candidate vaccines being superseded due to strategic decisions (Ad26.RSV.preF),47 or failure to meet one of the primary endpoints (MVA-BN-RSV).48 Nanoparticle-based vaccines were designed to induce a robust neutralizing antibody response and have shown promising results in preclinical and early clinical studies.49 A phase II clinical trial was initiated in June 2023 to determine the efficacy of IVX-A12, a nanoparticle-based vaccine for immunizing older adults with RSV and human metapneumovirus.50 The phase I trial of a live attenuated vaccine RSV-MinL4-0 showed a humoral and cellular immune response similar to wild-type infection in non-human primates.51 The chimeric live virus vaccine CPI-RSV-F (BLB201) is currently in phase I trial.52 Data from phase II+III trial of mRNA-1345 (Moderna), a single-dose mRNA candidate encoding stabilized RSV pre-F protein showed vaccine efficacy of 84% against RSV-LRTD with at least two signs or symptoms, 82% against the disease with at least three signs or symptoms, and 68% against RSV-associated acute respiratory disease.53 mRNA-1345 efficacy was observed against RSV subtypes A and B and was not associated with evident safety concerns.53
In preterm and healthy-term infants, long-acting monoclonal antibodies against RSV have also shown outstanding results.54,55 Nirsevimab, an extended half-life monoclonal antibody against RSV, showed high efficacy in protecting healthy infants who were born preterm or at full term against hospital admission for RSV-lower respiratory tract infections (LRTIs) with a relative risk reduction of approximately 80%.56,57 Nirsevimab is also specifically indicated in infants with high-risk conditions including those with chronic respiratory conditions, although only during the first two years of life. It has been specifically studied in infants with bronchopulmonary dysplasia or chronic lung disease.56,58 In Galicia, Spain, the first region in the world to include nirsevimab in its immunization calendar, the uptake rate by the high-risk group was as high as 98%.59 Studies on nirsevimab impact using real-world data are outgoing. The first longitudinal study on nirsevimab impact reported effectiveness of 86.9% (95% CI: 69.1, 94.2) against severe RSV-LRTI requiring oxygen, 69.2% (95% CI: 55.9, 78.0) against all-LRTI hospitalizations, and 66.2% (95% CI: 56.0, 73.7) against all-cause hospitalizations in Galicia, and no severe adverse events related to nirsevimab were registered.60 Another study involving nine hospitals in Spain reported a pooled nirsevimab effectiveness against RSV-related LRTI hospitalizations of 84.4% (95% CI: 76.8, 90.0) using screening design, and 70.2% (95% CI: 38.3, 88.5) using a test-negative design.61 A third study, from Luxembourg, compared the 2023-2024 RSV season after nirsevimab implementation to the past RSV season and observed decreased rates of RSV-related LRTI hospitalizations of 38% in children younger than five years and 69% in infants under six months of age.62
Regarding maternal vaccination against RSV, a phase III clinical trial concluded that the RSVpreF vaccine was effective against medically attended severe RSV-associated lower respiratory tract illness in infants with 82%, and 69% efficacy, 90 and 180 days after birth, respectively.41 Real-world data on pregnant women's immunization campaigns against RSV in infants are still unavailable. In any case, maternal vaccination cannot specifically target newborns with CRD, being monoclonal antibodies as the only preventive option that can be specifically targeted to infants with CRDs.
SARS-CoV-2The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has infected 772,011,164 persons and led to 6,979,786 deaths worldwide as of November 16, 2023.63 Studies on the long-term impact of COVID-19 on respiratory morbidity is emerging, and available data so far indicate that COVID-19 acts as a risk factor for CRD exacerbation.64
Based on current evidence, people with COPD are at an increased risk of hospitalization for COVID-19 and may be at increased risk of developing severe disease and death.44
People with asthma do not appear to have a higher risk of acquiring COVID-19, yet there is great variability in asthma prevalence among patients with COVID-19 in different countries or regions.20,65 Several studies showed that the risk of COVID-19 death was higher in patients with asthma recently hospitalized with severe asthma or recently treated with systemic corticosteroids. A national incident cohort study in Scotland found that asthmatic adults who required at least two courses of corticosteroids in the past two years, or who were hospitalized for asthma are at a higher risk of hospitalization, ICU admission, or death after acquiring SARS-CoV-2 infection.66 Nevertheless, a nationwide cohort study in the UK concluded that the use of inhaled corticosteroids, within 2 weeks of admission with COVID-19, improves survival for asthma patients aged 50 years and older, but not for those with chronic pulmonary disease.67 The UK study also found that in patients aged 16 years and older, severe asthma was associated with increased mortality compared to non-severe asthma.67
Vaccination against COVID-19 could prevent respiratory failure events, which can be fatal in CRD patients.68 Therefore, international health agencies and national guidelines such as the American Lung Association, the Centers for Disease Control and Prevention (CDC), and the Spanish COPD Guidelines advocate for COVID-19 vaccination in chronic lung disease, COPD, and asthma patients to avoid disease exacerbations.17,69,70 To date, 11 vaccines against COVID-19 were granted emergency use listing by the WHO (supplementary Table S1).71 Most of the data available so far on COVID-19 vaccine effectiveness (VE) have been generated from general populations and estimates varied across the literature, likely due to heterogeneity in study design, settings, and the epidemiological characteristics of the pandemic. Kwok et al. estimated that in CRD patients, including those with COPD, asthma, and bronchiectasis effectiveness who received at least two doses of CoronaVac, the risk of being hospitalized for COVID-19 and developing respiratory failure is reduced by more than 80% as compared to unvaccinated CRD patients.72 They found similar results for CRD patients vaccinated with BNT162b2 (hospitalization VE=79%; respiratory failure VE=91%).72
VE declines with time, necessitating one or more booster dose schedules. Higher VE was estimated for booster doses, with the protection mainly pronounced against severe COVID-19 in the older adult population.73 In the 5-11-year child population, two meta-analyses estimated VE against Omicron infections which ranged between 42% and 45%,74,75 after receiving two doses of COVID-19 vaccine. The WHO Technical Advisory Group on COVID-19 Vaccine Composition strengthened that booster doses confer protection against SARS-CoV-2 strains, including Omicron, and encouraged updating vaccine antigen composition to enhance vaccine-induced immune responses to circulating SARS-CoV-2 variants.76 Likewise, the European Centers for Disease Control and EMA jointly recommended updating vaccines to target XBB strains (a subgroup of Omicron) and suggested adopting monovalent vaccines to protect against current dominant and emerging strains.77
Based on data from the COVID-19 vaccine tracker and landscape tool of the WHO, as of March 30, 2023, there were 183 candidate COVID-19 vaccines in the clinical phase and other 199 vaccines in the pre-clinical development.78 Most of the vaccines that are in the clinical phase use protein subunit (n=59; 32%), followed by RNA (n=43; 24%), non-replicating viral DNA (n=25; 14%); inactivated virus (n=22; 12%) and DNA (n=17; 9%).78 Of the 183 vaccines, 164 (90%) are injectable mainly intra-muscularly (150/164) and 101 (55%) are engineered to be administered in two doses.22 Specific details on each vaccine are available at the COVID-19 vaccine tracker and landscape.78
Influenza VirusInfluenza disease is a contagious, acute viral respiratory illness that may affect all age groups with presentations ranging from mild upper respiratory symptoms to severe cases resulting in hospitalization and/or death. In the northern and southern hemispheres, influenza causes seasonal epidemics of disease almost every winter. In tropical regions, influenza circulates continuously throughout the year, causing outbreaks more irregularly.79 Influenza infections are associated with exacerbation of CRDs such as pneumonia, respiratory failure, and COPD leading to higher respiratory morbidity and mortality rates.23,80 Using data collected between 1999 and 2015 on seasonal influenza, global estimates of influenza-associated mortality and morbidity indicate that each year, influenza causes between 290,000 and 650,000 respiratory deaths which is equivalent to 4.0–8.8 deaths per 100,000 individuals, and approximately five million hospitalizations for LRTIs.81,82
In Europe, seasonal influenza vaccination is recommended for risk groups, such as adults older than 50 or 65 years, depending on the country, and individuals with chronic medical conditions.83 A seasonal influenza vaccine protects against the influenza virus strains that are expected to dominate in the upcoming season. Most European countries also follow WHO recommendations in vaccinating pregnant women and some of them advise vaccinating healthy children aged between six months and five years.83 Though not effective against asthma exacerbations,84,85 owing to its cost-effectivity and the high risk of complications in chronic disease patients as well as treatment failure in children, the Spanish Guideline on the Management of Asthma (GEMA) recommends vaccinating children and adults with moderate and severe asthma against influenza.86 In the United States, the CDC additionally recommends vaccination for all persons aged 6 months or below without contraindications.87 The CDC also recommends the use of licensed, age-appropriate influenza vaccines.88,89 A meta-analysis of influenza VE in asthma patients showed that influenza vaccine prevented between 59% and 78% of asthma attacks leading to emergency visits and/or hospitalizations, however, the authors of that meta-analysis acknowledged certain limitations related to heterogeneity in participant recruitment, vaccine ascertainment, type of vaccines, outcome definitions, participant characteristics between included studies.90 Therefore, further research with well-defined outcomes and enough sample size is required to draw solid conclusions on influenza VE in asthma patients. Li et al. estimated that the overall effectiveness for preventing acute COPD exacerbation, pneumonia, and related hospitalization were respectively 70%, 59%, and 58% in Chinese patients vaccinated against influenza.91 A study including nearly two million persons during four influenza seasons in the US also found that influenza vaccination was associated with an important reduction in the risk of all-cause and specific-cause death during the most severe periods of influenza seasons (all-cause VE=75%, respiratory causes VE=76%, and pneumonia/influenza cause VE=82%); although a part of this protective effect could be attributed to healthy vaccinee bias as the reduced risk of death was also seen when no influenza was circulating (all-cause VE=30%, respiratory causes VE=32%, and pneumonia/influenza cause VE=51%).92
PneumococcusPneumococcus disease is a bacterial infection caused by Streptococcus pneumoniae (S. pneumoniae), or pneumococcus. Pneumococcal infections can cause pneumonia, meningitis, bacteraemia, sinusitis and otitis media, among others. There are>100 known serotypes of S. pneumoniae which distribution vary according to age, geographic regions, the presence of antibiotic resistance genes, as well as disease syndrome and severity.93
Children are the main reservoir of S. pneumoniae, with a prevalence of nasopharyngeal carriage ranging between 27% and 85%, and being more pronounced in low- and middle-income countries.93,94 The disease exacerbates in the presence of comorbidities and pneumococcal resistance genes.93,95 Pneumonia contributes to 14% of the deaths of children under five, with 740,180 related deaths registered in 2019.96 Considering the heavy burden of pneumococcal disease on public health, the WHO has recommended that all countries include pneumococcal conjugate vaccine (PCV) in their routine pediatric immunization calendar since 2007.93 Nonetheless, research showed that pediatric vaccination is not enough to control pneumococcus disease burden in adults, especially against mucosal infections.19 In addition to international vaccination guidelines such as that of the CDC, national vaccination guidelines have been implemented globally, though vaccination recommendations differ between countries in terms of age, risk groups, vaccine type, and dosing and timing between doses.97
Adult COPD patients have a higher risk of being hospitalized with community-acquired pneumonia and invasive pneumococcal disease than persons with other chronic lung diseases.98–101 The risk of community-acquired pneumonia is 18-fold higher in hospitalized COPD patients aged 40 compared with COPD-free patients.102 An association between a history of asthma and an increased risk for invasive pneumococcal disease has been also suggested.103,104 These findings suggest a potential benefit of community-acquired pneumonia prevention strategies. Therefore, the Global Initiative for Chronic Obstructive Lung Disease 2023 guidelines recommend vaccination against pneumococcus for COPD patients to prevent severe disease.105 The US Advisory Committee on Immunization Practices had also included asthma as an indication for pneumococcal vaccination in 2010.103,104 In addition, the Green Book 2023 (UK), included among the clinical risk groups recommended for pneumococcal disease vaccination, CRD patients with COPD, including chronic bronchitis and emphysema; and such conditions as bronchiectasis, cystic fibrosis, interstitial lung fibrosis, pneumoconiosis, and bronchopulmonary dysplasia; children with respiratory conditions caused by aspiration, or a neurological disease with a risk of aspiration; as well as those with severe asthma requiring continuous or frequently repeated use of systemic steroids.106 CRD patients are also categorized as risk groups for pneumococcal vaccination in CDC international guidelines as well as in many other national guidelines including those of Australia, Canada, France, Germany, New Zealand, and Spain, among others.19,97,107 As is the case for the influenza vaccine, pneumococcal vaccination has not shown definitive efficacy in the prevention of asthma exacerbations, nonetheless, it is a cost-effective approach, and given that the asthma population has a higher risk of invasive pneumococcal disease than non-asthma patients, pneumococcal vaccination is recommended in severe asthma patients.86
Two types of vaccines are available against pneumococcal diseases: pneumococcal polysaccharide vaccines (PPSV) and pneumococcal conjugate vaccines (PCV). To date, only one PPSV is available, the PPSV23 vaccine which acts against 23 bacterial serotypes causing pneumococcal disease. This vaccine is currently recommended only in sequential schedules following PCVs administration in the elderly population as well as for children older than two who are at high risk of the disease.108 Findings on the clinical efficacy of PPSV23 against community-acquired pneumonia are inconsistent.109 A meta-analysis suggested that PPSV23 could offer relative protection, for a limited duration, against invasive pneumococcal disease as well as invasive pneumococcal pneumonia by any serotype in the elderly, however, relevant concerns regarding the inclusion criteria and quality of data computed in that metanalysis have been raised.110
As compared to PPSV, PCVs induce cell immune response and have an impact on mucosal disease, namely pneumonia, The currently available pneumococcal conjugate vaccines include the 10-valent (PCV10), the 13-valent (PCV13), the 15-valent (PCV15), and the 20-valent (PCV20) vaccines; with the numbers representing the S. pneumoniae serotypes against which these vaccines protect.111 Results from several clinical trials established the immunogenicity of PCV13, PCV15 and PCV20 in adults.112–114 Simulation studies point-out a reduced incidence and mortality in adults through protection with PCV20 in comparison to PPSV23 have been reported.115,116
Given the advantages of PCVs over PPSV23, specifically, efficacy on non-invasive pneumococcal disease and immune cell-response stimulation, with the arrival of higher-valency PCVs, current international and national recommendations on vaccination of older adults such as that of the CDC, NEP in Spain, JCVI in UK, are switching to only PCV20 or sequential PCV13 or PCV15+PPV23 approach.19,97,109,117 The need for booster dose schedules and the characteristics and intervals of these additional doses in people who had been previously vaccinated with PCV13 and/or PPSV23 should be also determined.19,118
New PCV vaccines that encompass more serotypes and represent a new technology with a more robust immune response such as 24-valent PCV and V114 are currently under development.109 Findings from a US-based phase 1/2 clinical trial on V116, an investigational 21-valent PCV, were made available in 2023 and showed the vaccine was well tolerated, with a safety profile similar to PPSV23 and licensed PCVs.119 A third PCV candidate vaccine, PCV30+is currently in preclinical development.120
PertussisPertussis, also known as whooping cough, is a highly contagious respiratory infection caused by the bacterium Bordetella that can target individuals of all ages, but its effect is mainly pronounced in infants and low- and middle-countries.121–123 Pertussis affected 151,000 individuals worldwide in 2018.121 WHO recommended in its position paper - September 2015 to start vaccinating infants at 6-8 weeks of life.124 CDC recommends vaccinating people of all ages, including pregnant women, against pertussis, with recommendations for the vaccine type varying according to age.122 CDC vaccination strategy has been adopted by many countries as a preventive measure against pertussis, while healthcare workers, especially those who are in contact with children under 12 months of age, are prioritized.125
In the context of CRDs, emerging research suggests that pertussis contributes to respiratory disease exacerbation such as in the case of COPD.126 Therefore, Respiratory Prevention Experts recommend vaccinating adults with chronic disease and a higher risk of pertussis complications due to immunosuppression, COPD, or diabetes mellitus.127 Despite high vaccination coverage, pertussis remains an endemic disease that reoccurs every two to five years.128
Vaccines against pertussis are available as combinations with other antigens to protect against more than one disease.129 Two forms of vaccines against pertussis are currently used, the whole-cell vaccine (wP), and the acellular vaccine (aP). wP vaccines were first developed as suspensions of the entire Bordetella pertussis organism that has been inactivated, most of which are available as a combination vaccine, which contains antigens for diphtheria, tetanus, and pertussis (DTP). Due to the reactogenicity caused by wP vaccines, aP vaccines were then developed that contain only purified components of Bordetella.129 aP vaccines have gradually replaced wP vaccines in developed countries, but as 1) their development and production costs are substantially higher than that of wP vaccines, 2) the best aP vaccines have comparable protective efficacy to wP vaccines, and 3) the adverse events of the best aP and wP vaccines are relatively minor, wP remains the vaccine of choice in many developing countries.121 Studies on the cost-effectiveness of Tdap vaccines suggest that in asthma and COPD patients, Tdap boosters would importantly save direct costs, life years, and daily adjusted life years.130 Recently, Feredj et al. also reported an immunological efficiency of pertussis vaccination in the COPD population.131 Further information on pertussis vaccines is available in the WHO position paper on pertussis.129
Cost-effectiveness of Vaccination ProgramsGlobally, the cost-effectiveness of vaccination programs is well established, yet limited information is available about the specific cost savings in CRD patients.
In the context of RSV, the estimation of the cost-effectiveness associated with nirsevimab or maternal vaccine administration is crucial for the prioritization of any of the two immunization strategies. Given their novelty, related cost-effectiveness studies are still emerging. As nirsevimab is expected to be as highly effective as or even more than palivizumab it is expected to be cost-saving. The cost-effectiveness of using nirsevimab for infants aged less than 8 months born during or entering their first RSV season (at $445 per dose) was estimated to be $102,811 per quality-adjusted life year.132 In Canada, Shoukat et al. undertook cost-effectiveness and budget impact analyses of nirsevimab and maternal vaccination and demonstrated that the more expansive the target population, the greater the reduction in overall disease burden.133 They estimated that using a willingness-to-pay of CAD$50,000 per quality-adjusted life year gained, immunizing an entire birth cohort with nirsevimab would be cost-effective from a societal perspective for a price per dose of up to $290, with an annual budget impact of $83,978 for 1113 infants per 100,000 population.133 If a combined strategy of vaccinating pregnant women and immunizing only infants at high risk of severe disease is adopted, the cost-effectiveness would be lower ($49,473 per 100,000 population with a price per dose of $290 for nirsevimab and $195 for RSVpreF vaccine), and infant mortality by 76%-85%, compared to a 78% reduction achieved through a nirsevimab-only program of the entire birth cohort.133 Though the Canadian study was well executed, it was associated with limitations related to the no assessment of other relevant immunization strategies and the comparison groups, as discussed by Li et al., 2024.134 Likewise, a multicenter European study, found that from a full societal perspective, including leisure time lost, the seasonal mAb plus catch-up program was cost-saving in Denmark, England, Finland, Italy and Scottland.135
Systematic reviews of the economic evaluation of COVID-19 vaccination found that vaccination programs would be cost-effective and even cost-saving compared to no vaccination at all, with vaccine efficacy ranging from 65% to 75%.136,137 Cost-effectiveness was reported even when the efficacy of vaccines largely varied across settings, was assumed to be relatively low, and when only a specific age cohort was targeted for vaccine administration.136 For instance, in Catalonia, Spain, it was estimated that the benefit/cost ratio of COVID-19 vaccination is 3.4 from a social perspective and 1.4 from a health system perspective.138
A systematic review of studies on adult vaccination in the United States and Canada found that the percentage indicating cost-savings was 56% for influenza, 31% for pneumococcal, and 23% for tetanus-diphtheria-pertussis vaccinations among outcomes assessing age-based vaccinations.139 A European study on the cost-effectiveness of high-dose vs. standard-dose influenza quadrivalent vaccine in elder adults in Belgium, Finland, and Portugal, found that the former contributes to a significant improvement in preventing influenza health outcomes while being cost-effective.140
Practical Recommendations and Conclusions- 1.
Respiratory infections highly contribute to CRD exacerbations, resulting in elevated morbidity and mortality rates. Vaccination against infectious diseases may thus avoid CRD exacerbations due to infections. In the last years, new vaccines that potentially protect against respiratory infections have been introduced, and the clinical recommendations have been updated to include CRD patients, especially COPD and asthma, among priority groups for vaccination.
- 2.
Based on the current knowledge of CRDs and the available vaccines and monoclonal antibodies, the authors of this report propose that CRD patients get immunized against RSV, influenza, SARS-CoV-2, pneumococcal, and pertussis. We rated the quality of evidence according to study design, giving randomized clinical trials, systematic reviews, and meta-analyses the highest quality (level A) and observational studies a decreasing order quality as follows: cohort studies (level B), test-negative design and case-control (level C), and before-after-study (level D).141 The authors also propose the following immunization schedule against respiratory infections in CRD patients:
- 3.
RSV: Nirsevimab is recommended for toddlers younger than two years of age with CRD. RSV vaccination for adults> 60 years. The need for a booster schedule is to be established. Elderly vaccination showed substantial efficacy against RSV-LRTD with level A evidence (phase III clinical trials). Nirsevimab also showed considerable efficacy in clinical trials (Level A evidence), and high effectiveness against RSV-related outcomes in a population-based cohort study (Level B evidence),60 a Test-Negative design study (Level C evidence),61 and a before-after study (Level D evidence).62
- 4.
SARS-CoV-2: Data on COVID-19 VE en CRD patients are limited, yet studies with level B (cohort studies) pointed to the high risk of hospitalization and death in patients recently hospitalized with severe asthma or recently treated with systemic corticosteroids.66,67 Level C evidence (case-control study) also reported COVID-19 VE in preventing hospitalization and respiratory failure in CRD patients with COVID-19.72 The standard vaccination schedule requires an initial immunization with the original strain and successive boosters. The boosters are preferred to be with an adapted vaccine updated to the circulating strains, although the frequency of booster administration remains to be established.
- 5.
Influenza vaccine: Different levels of evidence are available on influenza vaccine effectiveness against CRD exacerbation, with a meta-analysis on asthma exacerbation (level A lowered to B due to limitations in that meta-analysis), and a level B on COPD. Though current data suggest a significant benefit from influenza vaccination for CRD patients, further research is required. Therefore, in line with national and international guidelines, all chronic respiratory disease patients are recommended to get vaccinated against influenza yearly; the same applies to children with CRD. Depending on the age, a reinforced vaccine dose would be advisable.
- 6.
Pneumococcus: Data from levels A and B evidence indicate a substantially higher risk of pneumonia in CRD patients with COPD and asthma. Universal vaccination for children under five years with PCV15 or PCV20 and for individuals over 65 years with either PCV20 or PCV15+PPV23 is recommended. Individualized vaccination for at-risk individuals, including CRD, is advised irrespective of age.
- 7.
Pertussis: Limited data are available on pertussis and CRD exacerbation. Level A evidence indicates pertussis contributes to COPD worsening.126 A completed immunization against pertussis is recommended for all age groups. Booster dose schedules are to be considered in adolescents-young adults and specifically, in patients with chronic lung disease.
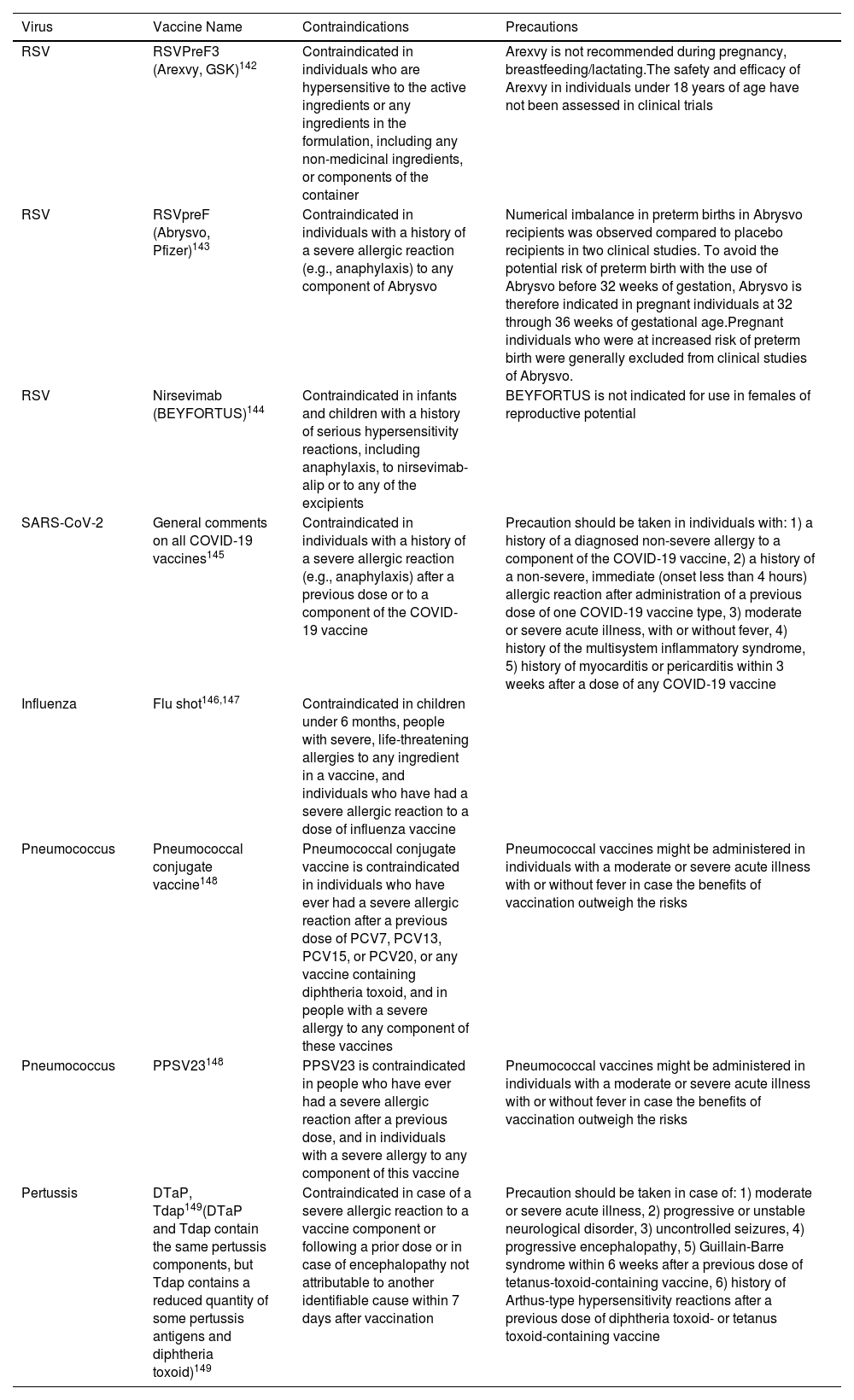
In any case, the specific contraindications and precautions for each of the recommended vaccines should be considered before administration (Table 2).
Summary of contraindications for vaccine administration and precautions in specific populations.
| Virus | Vaccine Name | Contraindications | Precautions |
|---|---|---|---|
| RSV | RSVPreF3 (Arexvy, GSK)142 | Contraindicated in individuals who are hypersensitive to the active ingredients or any ingredients in the formulation, including any non-medicinal ingredients, or components of the container | Arexvy is not recommended during pregnancy, breastfeeding/lactating.The safety and efficacy of Arexvy in individuals under 18 years of age have not been assessed in clinical trials |
| RSV | RSVpreF (Abrysvo, Pfizer)143 | Contraindicated in individuals with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of Abrysvo | Numerical imbalance in preterm births in Abrysvo recipients was observed compared to placebo recipients in two clinical studies. To avoid the potential risk of preterm birth with the use of Abrysvo before 32 weeks of gestation, Abrysvo is therefore indicated in pregnant individuals at 32 through 36 weeks of gestational age.Pregnant individuals who were at increased risk of preterm birth were generally excluded from clinical studies of Abrysvo. |
| RSV | Nirsevimab (BEYFORTUS)144 | Contraindicated in infants and children with a history of serious hypersensitivity reactions, including anaphylaxis, to nirsevimab-alip or to any of the excipients | BEYFORTUS is not indicated for use in females of reproductive potential |
| SARS-CoV-2 | General comments on all COVID-19 vaccines145 | Contraindicated in individuals with a history of a severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a component of the COVID-19 vaccine | Precaution should be taken in individuals with: 1) a history of a diagnosed non-severe allergy to a component of the COVID-19 vaccine, 2) a history of a non-severe, immediate (onset less than 4 hours) allergic reaction after administration of a previous dose of one COVID-19 vaccine type, 3) moderate or severe acute illness, with or without fever, 4) history of the multisystem inflammatory syndrome, 5) history of myocarditis or pericarditis within 3 weeks after a dose of any COVID-19 vaccine |
| Influenza | Flu shot146,147 | Contraindicated in children under 6 months, people with severe, life-threatening allergies to any ingredient in a vaccine, and individuals who have had a severe allergic reaction to a dose of influenza vaccine | |
| Pneumococcus | Pneumococcal conjugate vaccine148 | Pneumococcal conjugate vaccine is contraindicated in individuals who have ever had a severe allergic reaction after a previous dose of PCV7, PCV13, PCV15, or PCV20, or any vaccine containing diphtheria toxoid, and in people with a severe allergy to any component of these vaccines | Pneumococcal vaccines might be administered in individuals with a moderate or severe acute illness with or without fever in case the benefits of vaccination outweigh the risks |
| Pneumococcus | PPSV23148 | PPSV23 is contraindicated in people who have ever had a severe allergic reaction after a previous dose, and in individuals with a severe allergy to any component of this vaccine | Pneumococcal vaccines might be administered in individuals with a moderate or severe acute illness with or without fever in case the benefits of vaccination outweigh the risks |
| Pertussis | DTaP, Tdap149(DTaP and Tdap contain the same pertussis components, but Tdap contains a reduced quantity of some pertussis antigens and diphtheria toxoid)149 | Contraindicated in case of a severe allergic reaction to a vaccine component or following a prior dose or in case of encephalopathy not attributable to another identifiable cause within 7 days after vaccination | Precaution should be taken in case of: 1) moderate or severe acute illness, 2) progressive or unstable neurological disorder, 3) uncontrolled seizures, 4) progressive encephalopathy, 5) Guillain-Barre syndrome within 6 weeks after a previous dose of tetanus-toxoid-containing vaccine, 6) history of Arthus-type hypersensitivity reactions after a previous dose of diphtheria toxoid- or tetanus toxoid-containing vaccine |
As new vaccines and monoclonals are expected to be incorporated into the clinical arena in the following years, health authorities must develop an agile response to include these new products in the immunization calendar and bring them first to the at-risk population. Strategies at the organizational level and lessons from the COVID-19 pandemic such as the use of mass-vaccination sites, reaching patients and following them -up through mobile services, auditing of vaccination sites, and the compromise of the different stakeholders involved in vaccination, are crucial to improve vaccine uptake. Inadequate knowledge and clinically negative attitudes are challenging immunization strategies, especially since vaccination coverage remains below target levels established by WHO such as in the case of influenza or pneumococcal vaccinations among COPD patients. Therefore, the need for educational programs to improve the patients’ and healthcare professionals’ knowledge and attitudes on vaccine uptake should be assessed. The availability of homogenous and consistent clinical guidelines and recommendations from expert vaccination committees coupled with educational intervention measures, may indeed help to improve vaccination uptake in CRD patients.
Funding SourcesThis work was supported by consorcio Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CB21/06/00103; F.M-T), DIAVIR (Instituto de Salud Carlos III [ISCIII]/DTS19/00049/Cofinanciado FEDER; Proyecto de Desarrollo Tecnológico en Salud), Resvi-Omics (Instituto de Salud Carlos III [ISCIII]/PI19/01039/Cofinanciado FEDER), BI-BACVIR (PRIS-3; Agencia de Conocimiento en Salud [ACIS]-Servicio Gallego de Salud [SERGAS]-Xunta de Galicia; Spain), Programa Traslacional COVID-19 (ACIS-Servicio Gallego de Salud [SERGAS]-XUNTA de Galicia; Spain) and Axencia Galega de Innovacion ́ (GAIN; IN607B 2020/08-XUNTA de Galicia; Spain); and ReSVinext (Instituto de Salud Carlos III [ISCIII]/PI16/01569/Cofinanciado FEDER), Enterogen (Instituto de Salud Carlos III [ISCIII]/PI19/01090/Cofinanciado FEDER), OMI-COVI-VAC (PI22/00406/Cofinanced European Regional Development Fund), Grupos de Referencia Competitiva (IIN607A2021/05) and Axencia Galega de Innovación (GAIN; IN845D 2020/23-Xunta de Galicia; Spain).
Declaration of Potential Conflicts of InterestFederico Martinón-Torres has acted as principal investigator in randomized controlled trials of Ablynx, Abbot, Seqirus, Sanofi Pasteur MSD, Sanofi Pasteur, Cubist, Wyeth, Merck, Pfizer, Roche, Regeneron, Jansen, Medimmune, Novavax, Novartis and GSK, with honoraria paid to his institution. Federico Martinon-Torres reports a relationship with GSK Vaccines SRL that includes: consulting or advisory. Federico Martinon-Torres reports a relationship with Pfizer Inc that includes: consulting or advisory. Federico Martinon-Torres reports a relationship with Sanofi Pasteur Inc that includes: consulting or advisory. Federico Martinon-Torres reports a relationship with Janssen Pharmaceuticals Inc that includes: consulting or advisory. Federico Martinon-Torres reports a relationship with MSD that includes: consulting or advisory. Federico Martinon-Torres reports a relationship with Seqirus Pty Ltd that includes: consulting or advisory. The remaining authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Ther other authors declare no competing interests.