Mycobacterium malmoense is a gram-positive, acid-fast, slow-growing, non-chromogenic bacillus. It was described in 1977 in Malmö (Sweden) by Schröder and Juhlin. It was restricted to northern Europe but its incidence has increased globally in recent years.1 Here we present a case of infection by M. malmoense in a patient with adult cystic fibrosis (CF).
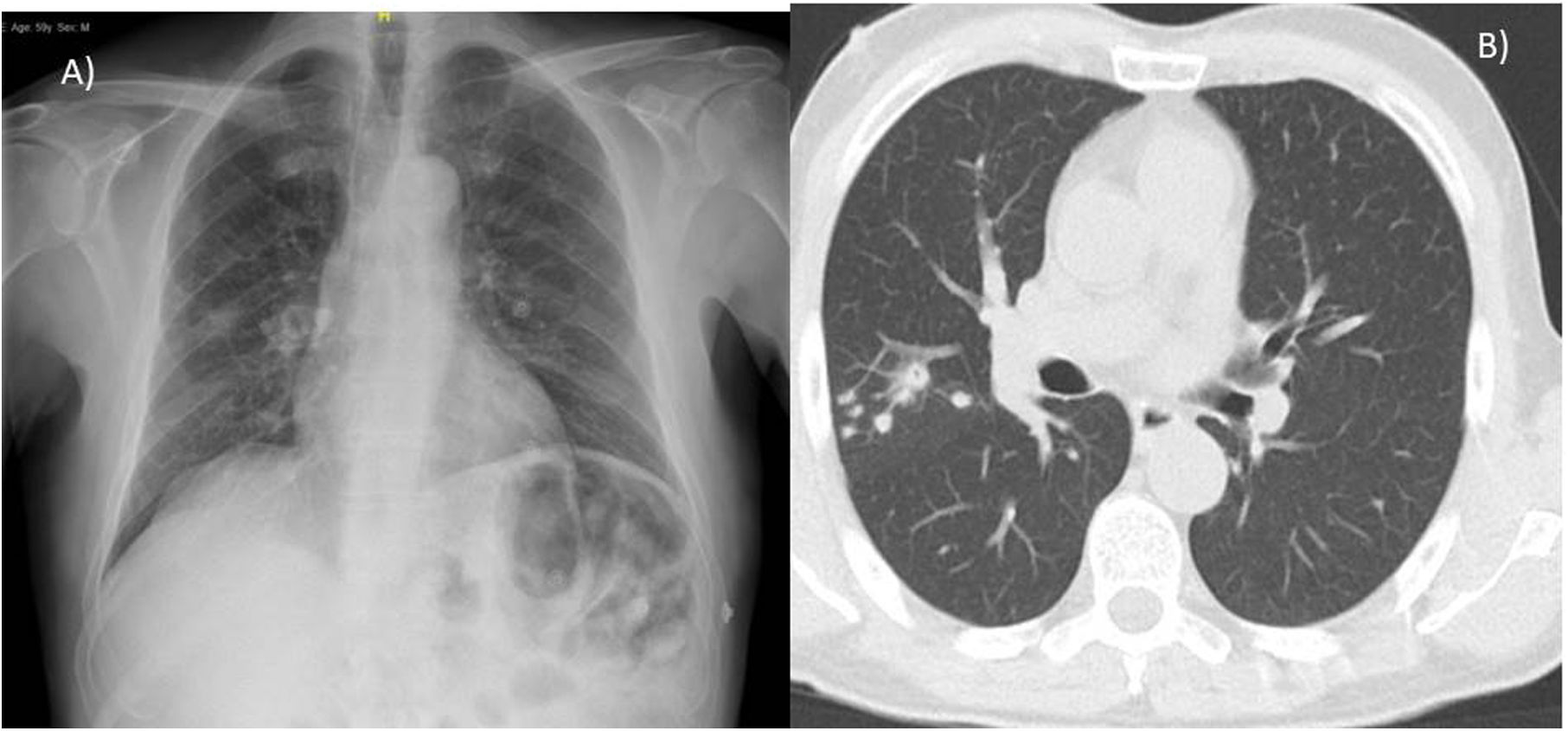
A 59-year-old male, with no toxic habits or history of lung disease, with a sister who died at 23 years of age due to CF. In the presence of non-specific febrile symptoms, a chest X-ray was requested, which revealed a pseudonodular infiltrate in the right upper lobe (RUL). Chest CT reported pulmonary nodule in RUL with adjacent nodules and centrolobular pulmonary micronodules (Fig. 1).
Bronchoscopy was performed and the bronchoalveolar lavage (BAL) cytology was benign. Examination of the specimens for direct acid-fast bacillus was negative. Eight weeks later, M. malmoense growth was observed in Lowenstein-Jensen culture of BAL. In the absence of respiratory symptoms, a wait-and-see approach was adopted until the antibiogram was obtained. In the sweat test, chloride concentrations were 124mmol/L (normal value <95mmol/L Macroduct system) and 115mmol/L (normal value <60mmol/L chlorimeter) and the genetic study was compatible with adult CF by showing two heterozygous mutations in CFTR gene (2789+5G>A y G85E). In spirometry, the FEV1/FVC index was 82% with an FEV1 of 107% and an FVC of 103%.
At one month, radiological control showed a spontaneous decrease of the lesions in RUL. Treatment was started with ethambutol (E)+isoniazid (I)+pyrazinamide (P)+rifampicin (R). Radiological resolution was observed after eight months of treatment. The patient did not present extrapulmonary manifestations of CF.
Pulmonary infection by M. malmoense represents its usual clinical form.1 It is prevalent in middle-aged men with a history of lung disease, toxic habits, haematological diseases or solid neoplasms.1,2
Its isolation does not imply disease in 20% of cases. The American Thoracic Society (ATS) diagnostic criteria for non-tuberculous mycobacteria (NTM) infection are less applicable to M. malmoense.2 Cavitated lesions >6cm are present in 30% of patients and the presence of hydroaerial levels or unilateral pleural effusion are more frequent in M. malmoense infection.3
In 2001, the first clinical trial was published to evaluate the therapeutic response in patients with pulmonary M. malmoense infection. They were treated with I, E and R or R and E for 2 years or 3 years, respectively, without showing significant differences in mortality or therapeutic failure but 4% died at five years due to the disease and the therapeutic failure/relapse rate was 10%.4 The addition of macrolides or fluoroquinolones does not provide significant additional benefit4 and amikacin is relegated to cavitary and/or severe disease.5
Sputum NTM is present in 13% of patients with CF. 20% of positive cultures meet ATS criteria. Mycobacterium avium complex, M. abscessus and M. massiliense account for 90% of these infections.5
The case presented here represents one of the few described in a patient with CF. There is no established treatment but there is good experience with ethambutol and rifampicin. However, the recurrence rate remains high, which raises the importance of individualizing treatment according to sensitivity/resistance tests and maintaining it for at least one year after the last culture conversion.
Conflict of InterestsThe authors state that they have no conflict of interests.