As stated in the Plan for the Prevention and Control of Tuberculosis in Spain,1 diagnosis and treatment of latent tuberculosis infection (LTBI) is a priority for achieving disease control in countries with low prevalence and high economic resources. Several international guidelines recommend that 85% of infected contacts should initiate treatment and 75% complete it2; furthermore, the World Health Organisation in its EndTB 2035 strategy includes among the measures to be taken, performing contacts screening in 90% of household contacts and completing treatment in 90% of those infected.3 However, the achievement of these objectives is sometimes complex due to various factors such as the characteristics of the infected people or physicians’ refusal to recommend LTBI treatment, which accounted for 36% of infected contacts in a recent series.4 With this study, our aim is to analyze whether these objectives are met in our country and the characteristics of infected contacts in whom LTBI treatment is not initiated.
In a prospective, observational, multicentre cohort study, contacts of patients diagnosed with tuberculosis (TB) between January 2018 and December 2019 in twelve centres of six autonomous communities in Spain were identified and studied after the diagnosis of the index case and followed up until the end of follow-up or treatment. All index cases and their contacts were consecutively included in the database of the SEPAR National Registry of the Integrated Tuberculosis and Non-Tuberculosis Mycobacteria Research Program (PII-TB &NMT),5 which was accessed through a username and password assigned to Group members. The inclusion of cases and contacts was carried out in accordance with the requirements expressed in the Declaration of Helsinki (Tokyo revision, October 2004) and the Organic Law on Data Protection 15/1999. The study was approved by the Ethics and Research Committee of all participating centres. The following definitions were used:
Indexcase6: a diagnosed case of TB in a person of any age in a specific household or other comparable setting in which others may have been exposed.
Contact6: that person who had a spatio-temporal coexistence relationship with the index case.
Secondarycase6: that person diagnosed with TB who was identified from the individuals studied as contacts.
Latent Tuberculous infection (LTBI)7: tuberculin skin test with an induration diameter equal to or greater than 5mm and/or positive IGRAs (Interferon-γ release Assays); QuantiFERON-TB GOLD in-Tube was used with a cut-off point of 0.35IU/ml.
Comorbidity: presence in a person of one or more diseases at the time of diagnosis of LTBI.
Treatment completed8: completion of the full course of treatment prescribed, regardless of whether an extension was needed to complete the full time period.
The comparison of proportions between groups was performed using the chi-square test (χ2), with Fisher's bilateral test when the expected values were less than 5. To identify associations between clinical and demographic factors and non-initiation of LTBI treatment, odds ratios and confidence intervals were reported. A p value of less than 0.05 was considered significant.
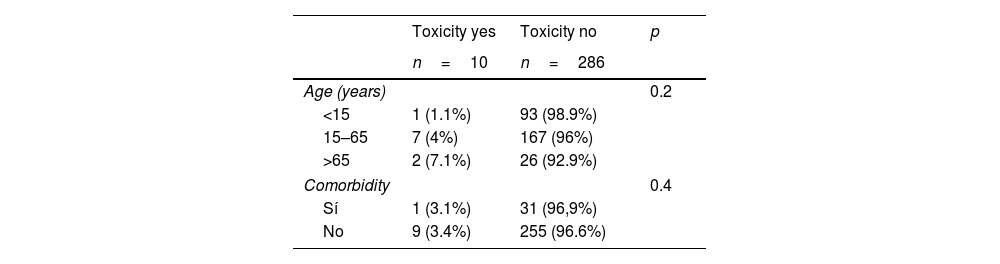
1035 contacts of 265 index cases were included, in 341 (33%) of whom latent tuberculosis infection was diagnosed: 183 men (53.6%) and 158 women (46.4%) with a mean age of 40.31±18.67 years old. LTBI treatment was recommended in 308 (90.3%), 12 refused to perform it, it was initiated in 296 (86.8%) and 262 (88.5%) completed it. The regimens used were isoniazid+rifampin for 3 months in 157 (53%), isoniazid for 6 months in 124 (41.9%), rifampin for 4 months in 5 (1.7%) and isoniazid for 9 months in 10 (3.4%), with compliance rate of 91.1%, 89.1%, 80% and 70% respectively. LTBI treatment induced toxicity in 10 subjects (3.3%) (hepatotoxicity in 9 and skin reaction in 1), in 3 treatment was discontinued; Table 1 shows their distribution according to age and presence of comorbidity.
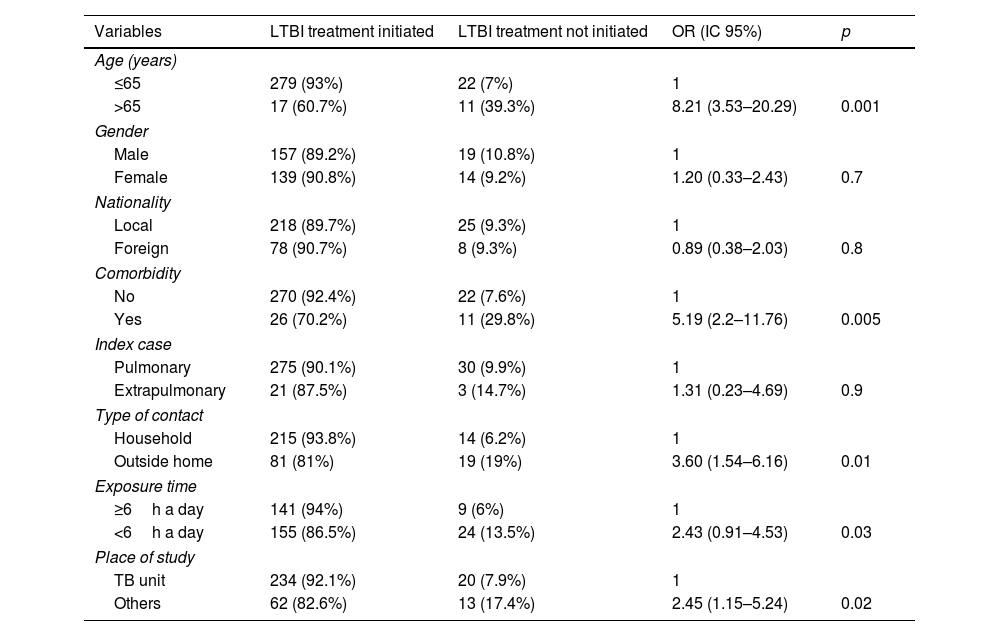
LTBI treatment was not initiated in 33 patients (9.7%) and it was significantly related to: age>65 years (p=0.001), presence of associated diseases (p=0.005), exposure outside the home (p=0.01), intensity of contact less than 6h per day (p=0.03) and study outside of specialized TB units (p=0.02), as shown in Table 2.
Failure to initiate LTBI treatment and related factors.
| Variables | LTBI treatment initiated | LTBI treatment not initiated | OR (IC 95%) | p |
|---|---|---|---|---|
| Age (years) | ||||
| ≤65 | 279 (93%) | 22 (7%) | 1 | |
| >65 | 17 (60.7%) | 11 (39.3%) | 8.21 (3.53–20.29) | 0.001 |
| Gender | ||||
| Male | 157 (89.2%) | 19 (10.8%) | 1 | |
| Female | 139 (90.8%) | 14 (9.2%) | 1.20 (0.33–2.43) | 0.7 |
| Nationality | ||||
| Local | 218 (89.7%) | 25 (9.3%) | 1 | |
| Foreign | 78 (90.7%) | 8 (9.3%) | 0.89 (0.38–2.03) | 0.8 |
| Comorbidity | ||||
| No | 270 (92.4%) | 22 (7.6%) | 1 | |
| Yes | 26 (70.2%) | 11 (29.8%) | 5.19 (2.2–11.76) | 0.005 |
| Index case | ||||
| Pulmonary | 275 (90.1%) | 30 (9.9%) | 1 | |
| Extrapulmonary | 21 (87.5%) | 3 (14.7%) | 1.31 (0.23–4.69) | 0.9 |
| Type of contact | ||||
| Household | 215 (93.8%) | 14 (6.2%) | 1 | |
| Outside home | 81 (81%) | 19 (19%) | 3.60 (1.54–6.16) | 0.01 |
| Exposure time | ||||
| ≥6h a day | 141 (94%) | 9 (6%) | 1 | |
| <6h a day | 155 (86.5%) | 24 (13.5%) | 2.43 (0.91–4.53) | 0.03 |
| Place of study | ||||
| TB unit | 234 (92.1%) | 20 (7.9%) | 1 | |
| Others | 62 (82.6%) | 13 (17.4%) | 2.45 (1.15–5.24) | 0.02 |
OR: odds ratio. CI: confidence interval.
In our series of Spanish centres, the percentage of patients initiating and completing treatment for latent tuberculosis infection meets the requirements of the international clinical guidelines and almost equals the World Health Organisation's recommendations. This proportion is significantly higher than reported in other series in our country carried out in specific geographical areas.9–11 and in studies developed in other countries with a low prevalence of the disease, such as the United States of America12 and Canada13 where 42–67.9% initiated therapy and 66.1–76% completed it. The high percentages found in our study can be explained by various factors, but we think it is important to emphasize that all the researchers were tuberculosis experts with regular involvement in specialized tuberculosis units, which have been shown to improve initiation and compliance treatment rates.14,15 Likewise, as the Spanish Health System is public and offers universal coverage, it would facilitate patient access to healthcare resources, which would undoubtedly also contribute to the final result being adequate.
On the other hand, in our study, older age, the presence of comorbidity, exposure outside the home, its lower intensity, and if the study was not performed in TB units, were all significantly associated with the possibility of not initiating LTBI treatment. In a recent series,16 which included 1047 infected contacts, in 54% of whom treatment was not initiated, which was related to age over 60 years and immigrant status, but not to the place of exposure. Similarly, in another study,12 older age and female gender were the variables associated with the likelihood of not initiating treatment, which represented 32% of the sample. Therefore, it appears that the common factor for physicians in making this decision would be age, which is likely influenced by the higher risk of developing pharmacological toxicity, as has already been shown in studies on tuberculosis patients,17 and the shorter life expectancy to develop disease. This attitude is reasonable, given that it is a preventive treatment. However, in our study this association between age and toxicity was not appreciated; thus, although the sample size does not allow us to draw definitive conclusions, it seems that age alone, in the absence of other factors, should not be a limiting factor for not initiating LTBI treatment, taking into account the health benefit that the intervention would produce, in this case lowering the risk of developing tuberculosis, which in infected contacts can reach 15%.18
Our study has limitations. Because this is an observational study involving several centres and researchers, it is possible that some of the variables were not collected correctly, implying a selection bias. However, as previously stated, all the researchers were experts in tuberculosis, with regular involvement in PII-TB&NMT, which we believe had a positive influence on the proper data collection and reduced this risk without compromising the validity of the results obtained.
We conclude that the recommended objectives for initiating and completing latent tuberculosis infection treatment are met in our setting. Treatment initiation is less likely with older age, shorter exposure time, if it occurs outside the home and the presence of associated diseases.
AuthorshipJosé Antonio Gullón Blanco: conception and design of the study: acquisition, analysis and interpretation of data, and writing of the article
José-María García-García and Teresa Rodrigo Sanz: critical review of the intellectual content, final approval of the version presented
Eva Tabernero Huguet: final approval of the version presented
Working group of the Integrated Tuberculosis and NMT Research Program (PII-TB & NMT): collection and contribution of data.
FundingThis study was funded by Ministry of Economy and Competitiveness-Carlos III Health Institute through FEDER Funds PI17/00724 grant and by the “Spanish Society of Pneumology and Thoracic Surgery” (SEPAR) through grant 378/2017.
Conflicts of interestThe authors have no conflicts of interest to disclose
Isabel Mir Viladrich (Pneumology, Hospital Son Llatzer, Palma De Mallorca). María Somoza-González (Pneumology, Consorcio Sanitario de Terrassa, Terrassa, España). Luis Anibarro and Christian Anchorena (Internal Medicine, Complexo Hospitalario de Pontevedra, Pontevedra). Ángel Domínguez-Castellano (Infectious Diseases, University Hospital Virgen Macarena, Sevilla,). Antón Penas-Truque (Pneumology, University Hospital Lucus Augusti, Lugo). Silvia Dorronsoro-Quintana (Pneumology, Hospital of Zumárraga, Zumárraga). Juan-Francisco Medina-Gallardo (Pneumology, University Hospital Virgen del Rocio, Sevilla). Lander Altube-Urrengoetxea (Pneumology, University Hospital of Galdakao, Galdakao). María Otero-Santiago (University Hospital of A Coruña, A Coruña). Manuel Angel Villanueva Montes and Concepción Rodríguez-García (Pneumology, University Hospital San Agustín, Avilés). Juan Rodríguez-López (Pneumology Hospital Grande Covián, Arriondas).