Pulmonary rehabilitation (PR) is recommended prior to bronchoscopic lung volume reduction (BLVR) procedures to optimize patient outcomes. However, there's a lack of clear guidance on PR content. The aim of our study is to examine the effect of adding inspiratory muscle training (IMT) to standard PR before BLVR on exercise capacity, dyspnea, fatigue level and quality of life.
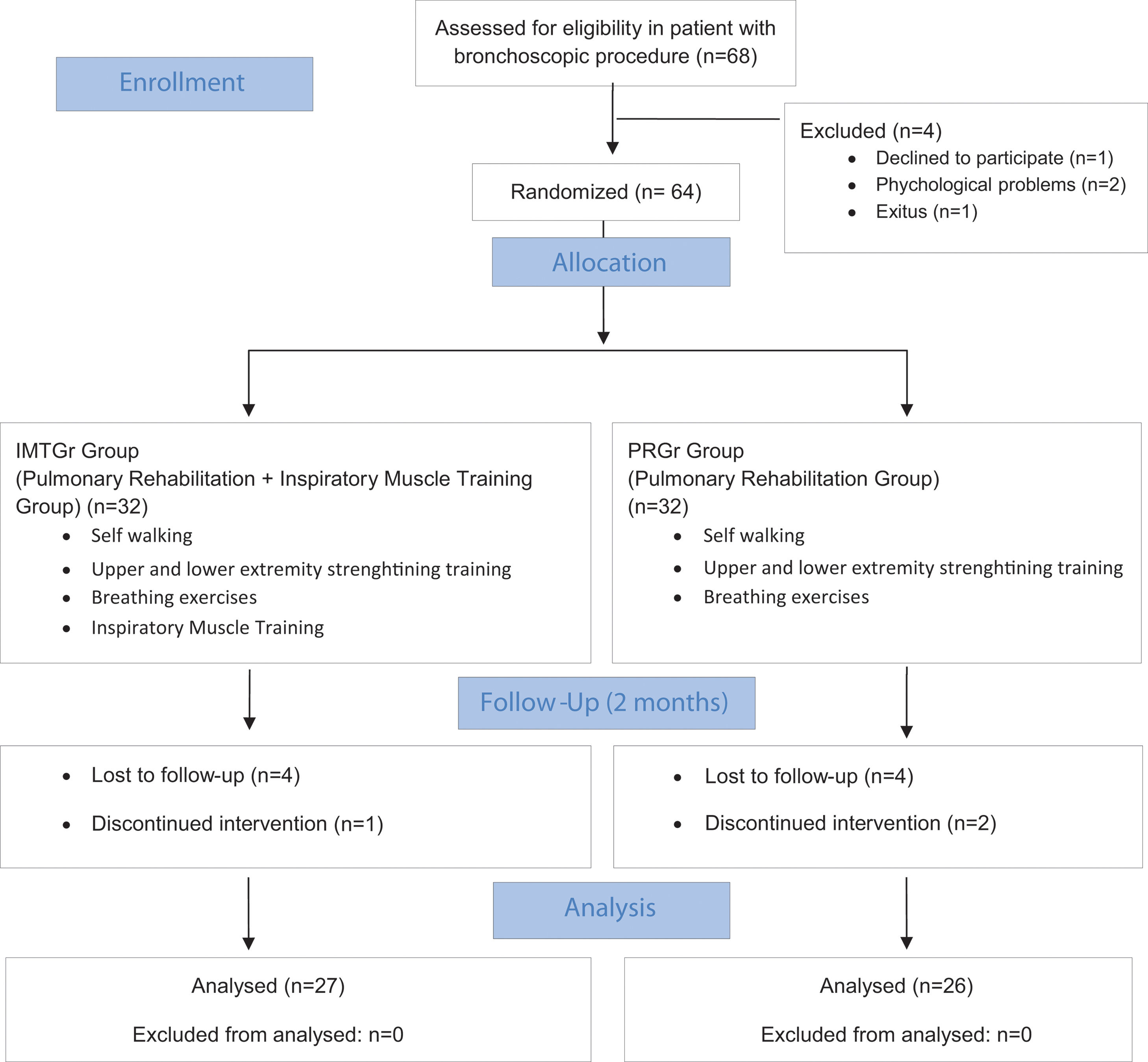
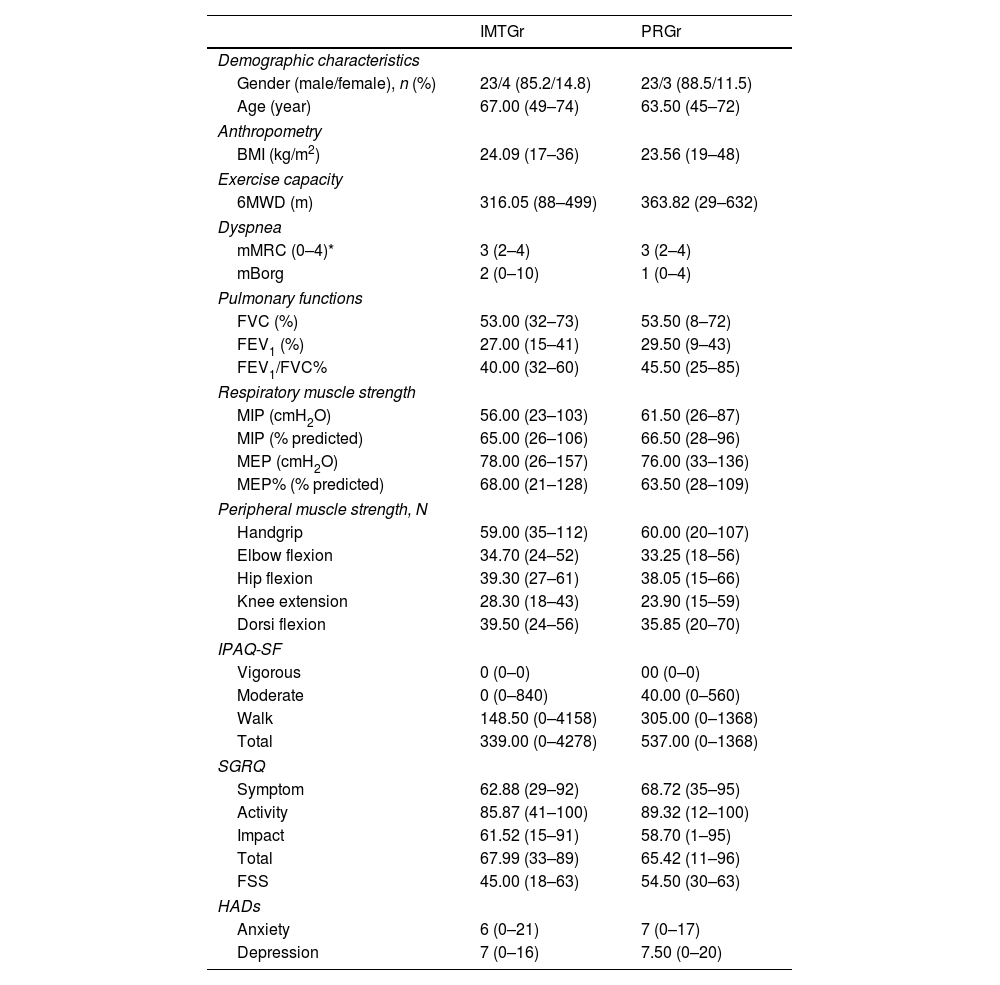
MethodsSixty-four patients were randomly assigned to either the PR Group (PRGr) or the PR with IMT group (IMTGr). Both groups underwent an 8-week standard PR program, including breathing exercises, muscle strengthening, and walking. Additionally, IMTGr received IMT sessions. Outcome measures comprised six-minute walking distance (6MWD), maximal inspiratory and expiratory pressures (MIP, MEP), peripheral muscle strength, modified Medical Research Council dyspnea score, fatigue symptom scale, spirometric parameters, Saint George Quality of Life Questionnaire (SGRQ), International Physical Activity Questionnaire Short Form (IPAQ-SF), and Hospital Anxiety and Depression Scale.
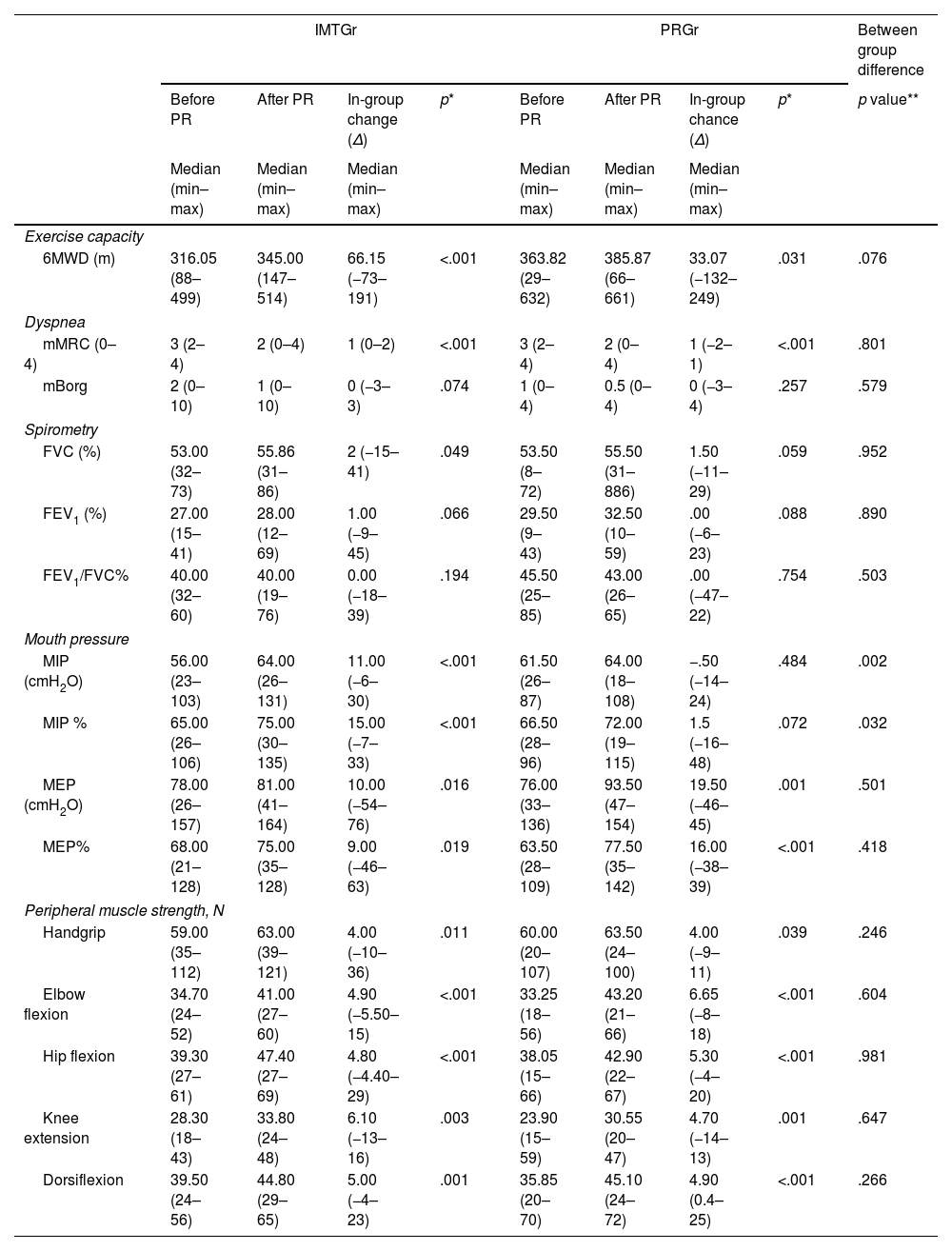
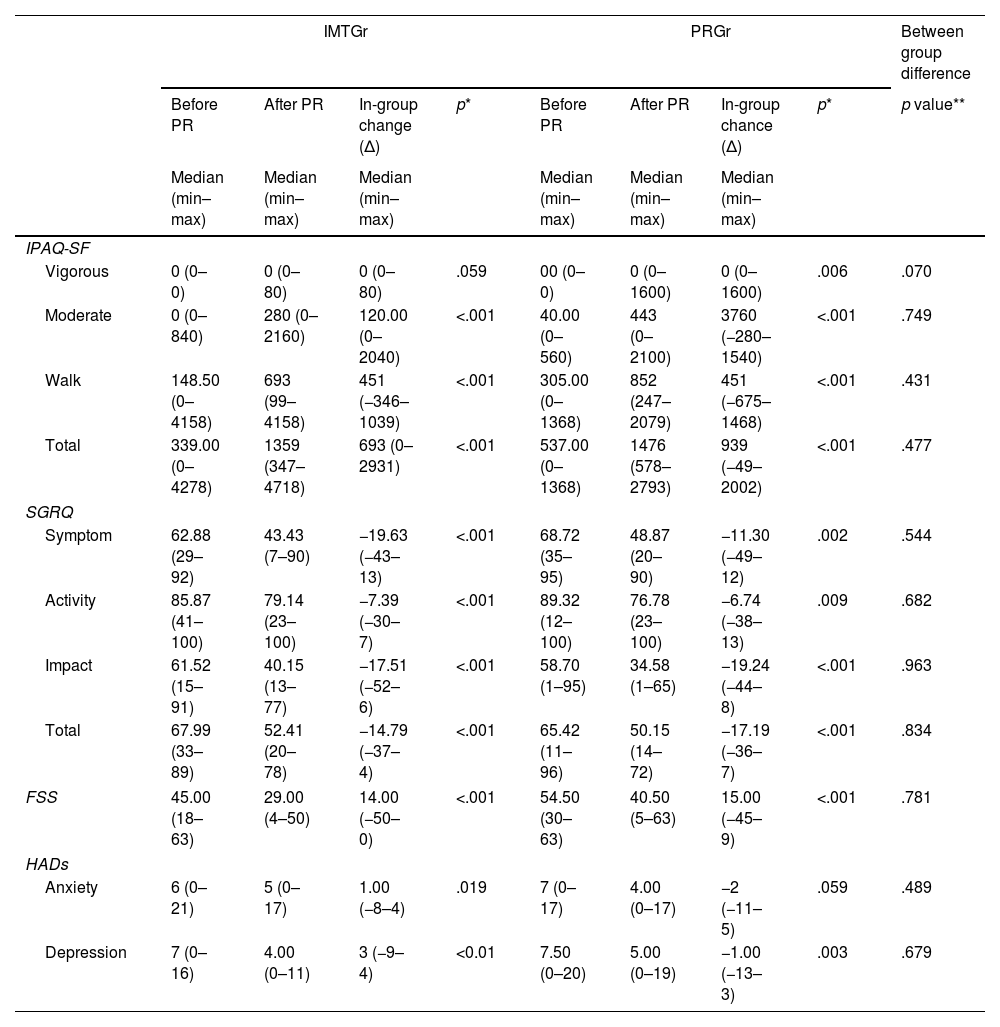
ResultsOur study found no significant difference in exercise capacity improvement between IMTGr and PRGr. However, IMTGr showed significant improvement in MIP compared to PRGr. Both groups experienced improvements in dyspnea, fatigue, and depression scores, as well as enhancements in 6MWD, MEP, peripheral muscle strength, IPAQ-SF and SGRQ scores.
ConclusionAdding IMT to PR did not show a significant difference between groups among BLVR-eligible patients. However, improved respiratory muscle strength may have positive clinical implications. Further research is needed to explore short and long-term effects.