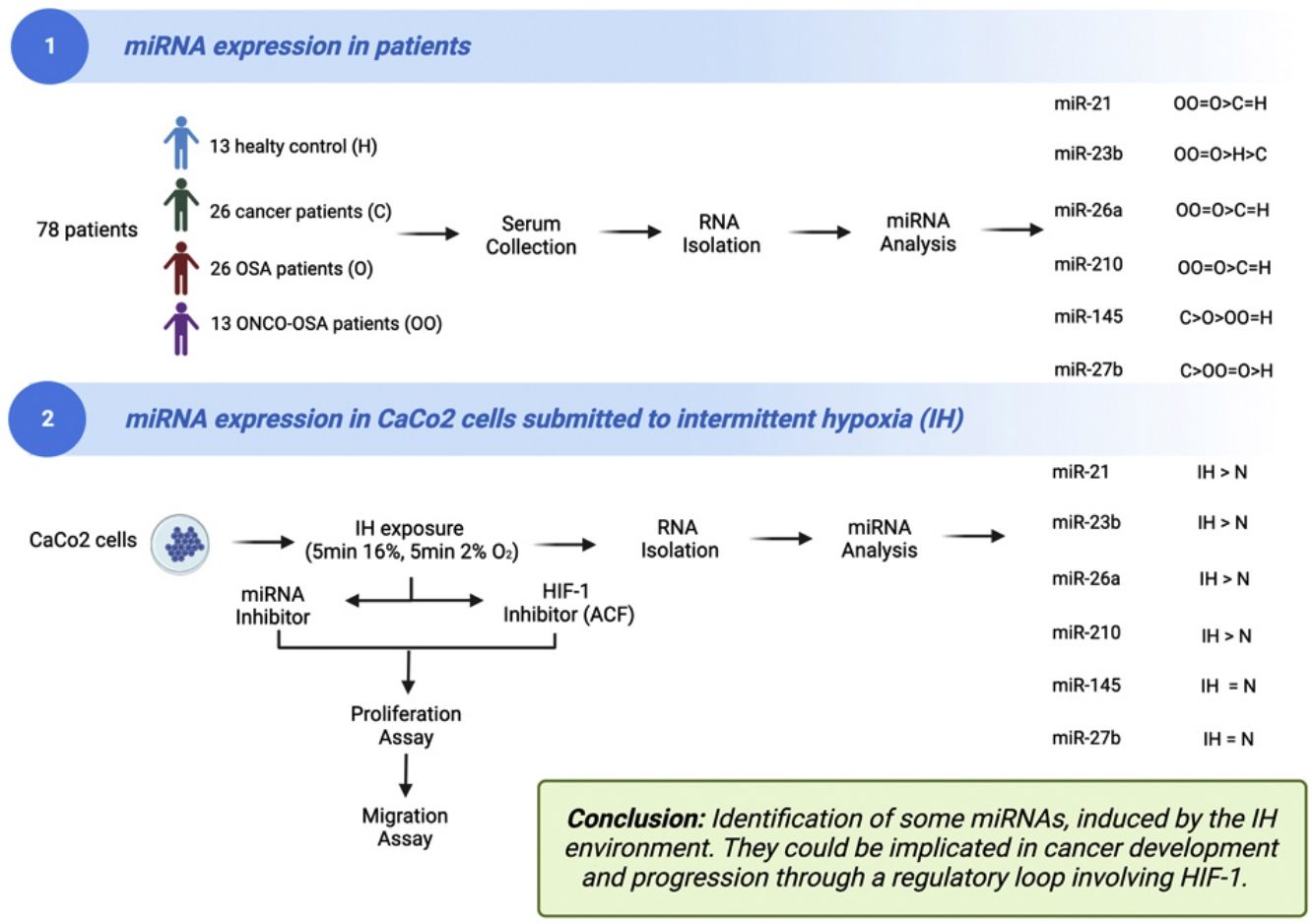
There is still a debate for the link between obstructive sleep apnoea (OSA) and cancer. The mechanisms underlying this causality are poorly understood. Several miRNAs are involved in cancer development and progression with expression being influenced by hypoxia.
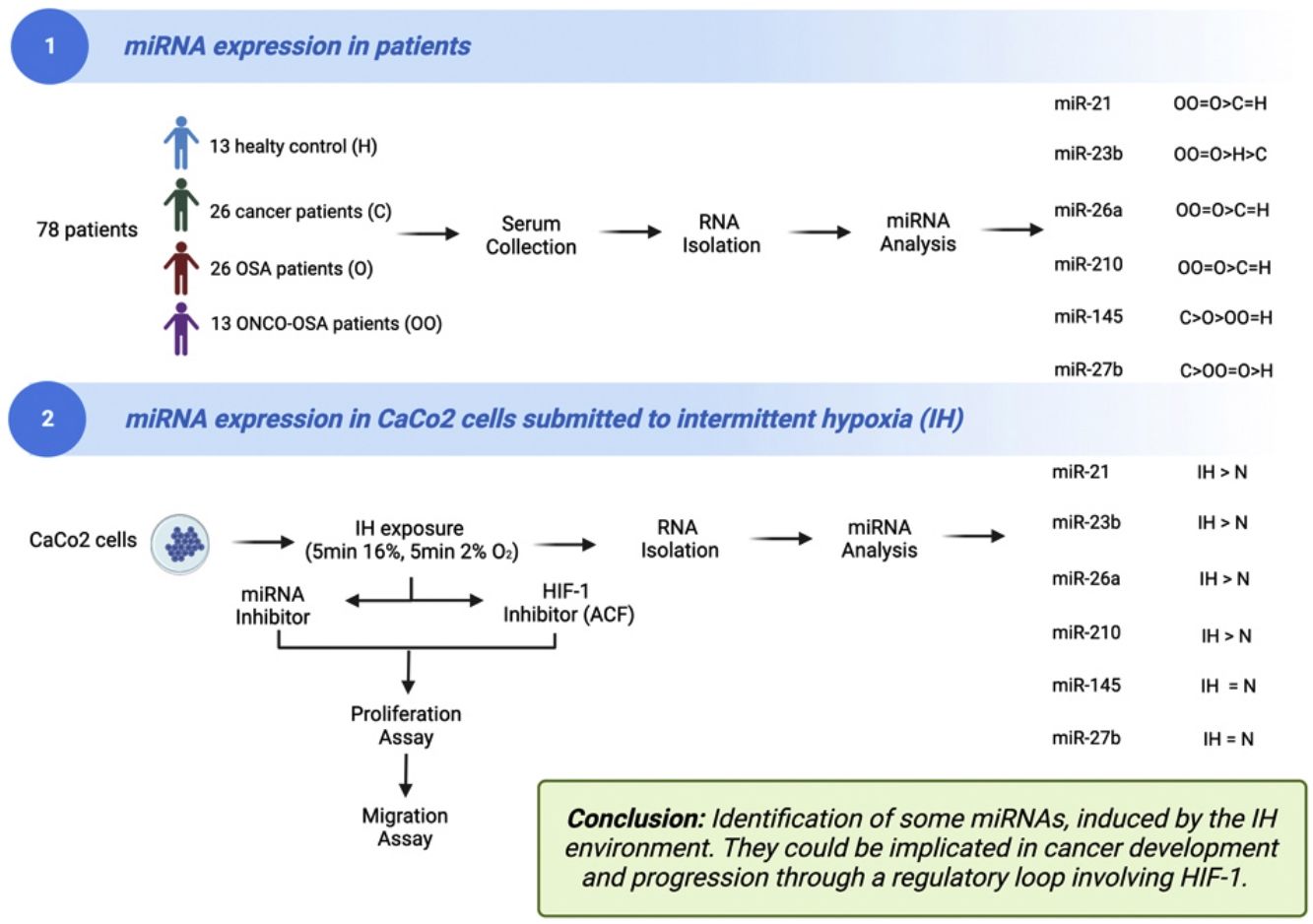
The aims of this work were (i) to compare miRNAs expression in controls versus patients affected by OSA without or with cancer (ONCO-OSA) and (ii) in colorectal cancer cells exposed to intermittent hypoxia (IH), to evaluate miRNAs impact on tumor progression in vitro.
MethodsWe detected miRNAs by qRT-PCR in patients’ sera and in CaCo2 cells exposed to 2–32h of IH with or without acriflavine (ACF), a HIF-1 inhibitor. Viability and transwell invasion test were applied to investigate the proliferation and migration of CaCo2 exposed to IH and treated with miRNA inhibitors or acriflavine. HIF-1α activity was evaluated in CaCo2 cells after IH.
ResultsThe levels of miR-21, miR-26a and miR-210 increased in OSA and ONCO-OSA patients compared to controls. MiR-23b increased in ONCO-OSA patients, and miR-27b and miR-145 increased in OSA but not ONCO-OSA patients. MiR-21, miR-26a, miR-23b and miR-210 increased in cells after IH. IH stimulated cell proliferation and migration. This effect was reduced after either miRNA inhibition or acriflavine treatment. MiRNA inhibition reduces HIF-1α gene expression. Conversely, acriflavine reduced the expression of these miRNAs.
ConclusionsWe identified a signature of miRNAs, induced by the IH environment. They could be implicated in cancer development and progression through a regulatory loop involving HIF-1.
Obstructive sleep apnoea (OSA) corresponds to the repetitive occurrence of partial (hypopnea) or complete (apnoea) pharyngeal collapses leading to intermittent hypoxia and sleep fragmentation.1
OSA is suspected to be associated with cancer development and the progression.2 Recent epidemiological studies have suggested that incident cancer and mortality is higher in OSA patients. Indices of OSA such as apnoea–hypopnea index (AHI) and namely oxygen desaturation index (ODI) were linked with tumor progression.3
Intermittent hypoxemia and/or sleep fragmentation affect sympathetic tone, angiogenesis, inflammatory processes and immunoregulatory cells. This translates in transcriptional changes affecting oncogenic with more invasiveness and resistance to treatment.2 In particular, intermittent hypoxia (IH) is the key trigger for tumor development and progression4,5 by activating various physiopathological pathways.6
MicroRNAs (miRNAs) are small, non-coding RNAs which are involved in the regulation of several biological processes like cellular proliferation, apoptosis and cellular differentiation.7,8 Some microRNAs act as tumor or oncogenic suppressors or promoters, restricting or promoting tumor progression and angiogenesis.9 Local sustained hypoxia is a key feature of tumoral microenvironment and has been reported as upregulating expression of several miRNA.10,11
Intermittent hypoxia, a key landmark of OSA might participate to development and progression of cancer via different pathways compared to sustained hypoxia12 and is also modulating miRNAs expression.8,10
The hypoxia-inducible factor 1 (HIF-1) transcription factor, stabilized by hypoxia, activates a wide variety of genes which are fundamental to the adaptation of cells at low oxygen concentration. These target genes are involved cellular processes such as angiogenesis, glucose metabolism, survival, and cellular death. HIF-1 is recognized as a biomarker associated with cancer aggressiveness in OSA.6 Moreover, there is a family of miRNAs, called HRM for hypoxia-regulated microRNAs, activated by hypoxia in several tumor types such as breast and colorectal cancer.13
The objective of this study was to document intermittent hypoxia-related expression of some miRNAs associated with carcinogenesis and to decipher HIF-1 role in this process. We chose 6 microRNAs, miR-21, miR-23b, miR-26a, miR-27b, miR-145 and miR-210: (i) mir-21 is associated, in many types of cancer, with tumor growth and metastasis. It acts on the HIF-1α/VEGF signaling pathway and, through PTEN targeting, leads to the activation of AKT and ERK1/2 signaling pathways14; (ii) miR-23b, acting through VHL, is able to control HIF-1α/VEGF and β-Tcf-4 signaling pathways.15 In addition, it was dysregulated in various forms of cancer; (iii) miR-26a expression could be induced by hypoxia via a HIF-dependent mechanism and it was up-regulated during different kinds of tumors16; (iv) few data on miR-27b and hypoxia are currently available. However, it is known that OSA-associated intermittent hypoxia leads to oxidative stress and oxygen free radical (ROS) production. Oxidative stress and miR-27b, through the activation of nuclear factor NF-κB, generate an inflammatory cascade typical of patients with sleep apnea; (v) miR-145 is a direct target of HIF-1α, which is often down-regulated in several cancer types. Either HIF-1 and vascular endothelial growth factor (VEGF) are decreased by miR-145 overexpression17; (vi) miR-210 is the most studied hypoxia-induced miRNA in cancer research. In hypoxia, miR-210 is up-regulated in response to hypoxia-inducing factors (HIF). HIF-1α drives miR-210 overexpression and subsequent alteration of cellular processes, including cell cycle regulation, mitochondrial function, apoptosis, angiogenesis, and metastasis.18
We analyzed the expression of these 6 miRNAs in subjects affected by OSA without or with cancer (ONCO-OSA), and in colorectal carcinomatous cell lines (CaCo2) which were exposed to IH. HIF-1 and miRNA inhibitors were used to decipher their respective roles in cell proliferation and migration.
MethodsStudy populationIt was a collaborative work between University of Foggia-ITALY and the HP2 lab at Grenoble-Alpes University-FRANCE.
Written informed consent was obtained from all subjects and an ethical approval was obtained from two institutional review board (Ethics Committee, Policlinico Riuniti of Foggia, Italy and Comité de Protection des Personnes Sud-Est V, Grenoble, France). Protocols conformed to the principles of the Declaration of Helsinki.
A total of 78 blood samples from patients and healthy controls were evaluated: (i) healthy controls (n=13); (ii) cancer patients (n=26); (iii) OSA patients (n=26); (iv) patients with OSA and cancer (ONCO-OSA, n=13).
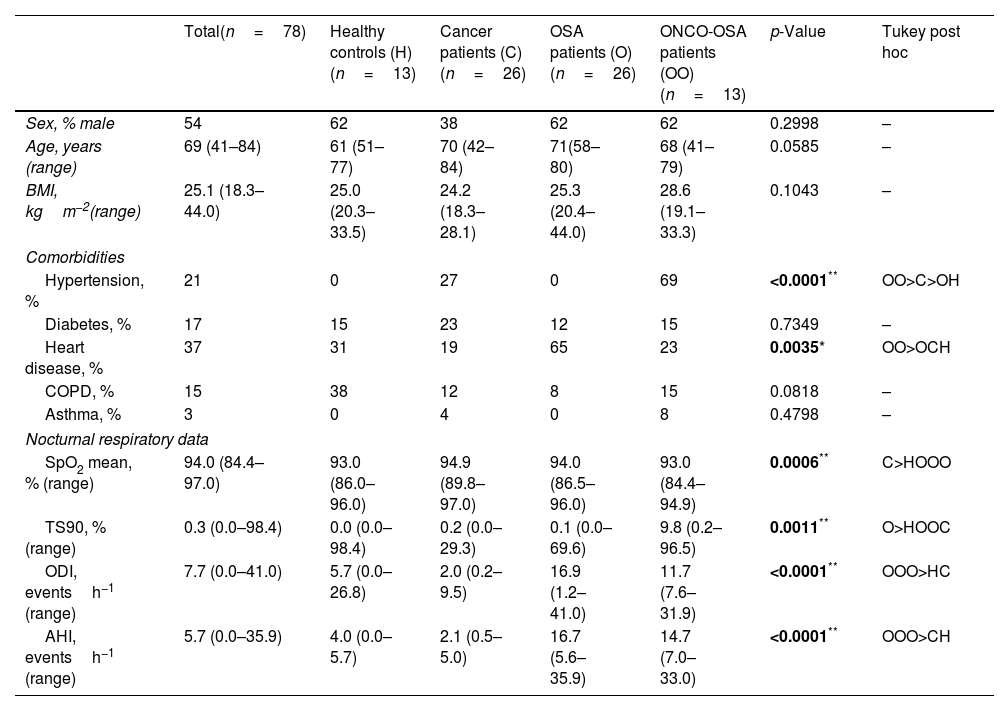
Participants’ baseline characteristics are reported in Table 1.
Clinical data of patients.
| Total(n=78) | Healthy controls (H)(n=13) | Cancer patients (C)(n=26) | OSA patients (O)(n=26) | ONCO-OSA patients (OO)(n=13) | p-Value | Tukey post hoc | |
|---|---|---|---|---|---|---|---|
| Sex, % male | 54 | 62 | 38 | 62 | 62 | 0.2998 | – |
| Age, years (range) | 69 (41–84) | 61 (51–77) | 70 (42–84) | 71(58–80) | 68 (41–79) | 0.0585 | – |
| BMI, kgm−2(range) | 25.1 (18.3–44.0) | 25.0 (20.3–33.5) | 24.2 (18.3–28.1) | 25.3 (20.4–44.0) | 28.6 (19.1–33.3) | 0.1043 | – |
| Comorbidities | |||||||
| Hypertension, % | 21 | 0 | 27 | 0 | 69 | <0.0001** | OO>C>OH |
| Diabetes, % | 17 | 15 | 23 | 12 | 15 | 0.7349 | – |
| Heart disease, % | 37 | 31 | 19 | 65 | 23 | 0.0035* | OO>OCH |
| COPD, % | 15 | 38 | 12 | 8 | 15 | 0.0818 | – |
| Asthma, % | 3 | 0 | 4 | 0 | 8 | 0.4798 | – |
| Nocturnal respiratory data | |||||||
| SpO2 mean, % (range) | 94.0 (84.4–97.0) | 93.0 (86.0–96.0) | 94.9 (89.8–97.0) | 94.0 (86.5–96.0) | 93.0 (84.4–94.9) | 0.0006** | C>HOOO |
| TS90, % (range) | 0.3 (0.0–98.4) | 0.0 (0.0–98.4) | 0.2 (0.0–29.3) | 0.1 (0.0–69.6) | 9.8 (0.2–96.5) | 0.0011** | O>HOOC |
| ODI, eventsh−1 (range) | 7.7 (0.0–41.0) | 5.7 (0.0–26.8) | 2.0 (0.2–9.5) | 16.9 (1.2–41.0) | 11.7 (7.6–31.9) | <0.0001** | OOO>HC |
| AHI, eventsh−1 (range) | 5.7 (0.0–35.9) | 4.0 (0.0–5.7) | 2.1 (0.5–5.0) | 16.7 (5.6–35.9) | 14.7 (7.0–33.0) | <0.0001** | OOO>CH |
Data are reported as median±min/max and were analyzed using Kruskal–Wallis test followed by Tukey post hoc test.
Participants were matched for age and BMI. Human fasted sera were collected and stored at −80°C until use.
Main data collected were as follows: comorbidities (hypertension, cardiovascular disease, diabetes, chronic obstructive pulmonary disease, asthma) and sleep studies data including apnoea–hypopnoea index (AHI), mean nocturnal SpO2, time with SpO2 lower than 90% (TS90) and oxygen desaturation index (ODI). OSA was defined as AHI>5.
Almost all ONCO-OSA and cancer patients had colorectal cancer (22 of 39 patients). Others exhibited ovarian cancer (n=4), breast cancer (n=4), lung cancer (n=2), gallbladder cancer (n=2), pancreatic cancer (n=4) or prostate cancer (n=1). Supplementary Table S1 depicts types of cancer affecting cancer and ONCO-OSA patients.
Cell cultureHuman colorectal adenocarcinoma cell lines (CaCo2) were purchased from American Type Culture Collection (ATCC). CaCo2 were maintained in Dulbecco's modified Eagle's medium (DMEM) 4500mg/l glucose (Gibco™ – Sigma–Aldrich, USA) supplemented with 10% fetal bovine serum (Sigma–Aldrich, USA), 100U/ml penicillin and 100μg/ml streptomycin (Gibco™ – Sigma–Aldrich, USA) at 37°C in a humidified atmosphere containing 5% CO2 and submitted to different duration of N or IH exposure.
An MTT assay was used to determine the cytotoxic effect of acriflavine (ACF, Gibco™ – Sigma–Aldrich, USA) on CaCo2 cell lines and 0.5μM ACF was considered optimal (data not shown). CaCo2 cells (1.5×105) were cultured in 6 well semipermeable plates pre-coated with type I collagen in complete medium for 24h. Just before exposing the cells to N/IH, the culture medium was replaced with DMEM supplemented with ACF 0.5μM to avoid toxicity.
Intermittent hypoxia exposureCaCo2 cells (1.5×105) were seeded in 6 well semipermeable plates (Zell-Kontakt Imaging FC plates, Germany) pre-coated with type I collagen in complete culture medium. Just before exposing the cells to N/IH, the culture medium was replaced with DMEM supplemented with 1% FBS, 100U/ml penicillin and 100μg/ml streptomycin.
Cultured CaCo2 cells were exposed to intermittent hypoxia (IH) as described before,19 alternating cycles of 5min of normoxia (16% PO2) and 5min of hypoxia (2% PO2) using a custom-made plate holder connected to two gas blenders (Gas Blender 100, MCQ Instruments, Rome, Italy) and located inside a standard cell culture incubator (SANYO, MCO-15AC). Control cells were exposed to constant normoxia (N, 16% PO2). PCO2 was maintained at 5% throughout exposure. The cells were subjected to this intermittent hypoxia system for up to 8h. A long exposure of 32h was also performed, consisting of 8h of IH followed by 16h of normoxia and another 8h of IH.
Isolation of RNARNA reverse transcription and expression of miRNA through q-PCRTransfection assaysCaCo2 cells were transfected with commercially available miRNA inhibitors for miR-21, miR-23b, miR-26a and miR-210 (anti-miR™ miRNA inhibitor: hsa-miR-21-5p, hsa-miR-23b-5p, hsa-miR-26a-5p and hsa-miR-210-3p – Invitrogen™) using Lipofectamine™ 2000 Reagent (Invitrogen™, Carlsbad, CA, USA) according to manufacturer's instructions.
RNA extraction and functional assays were performed 24h post-transfection. See Supplementary methods for more details.
Proliferation assaysTransfected CaCo2 cells (5×103) were seeded in 96-well semipermeable plates pre-coated with type I collagen and submitted to 32h of N/IH exposure. Cell viability was then assessed using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) staining as described in Supplementary methods.
Transwell migration assaysHIF-1α gene expressionFollowing miRNA inhibition, total RNA was extracted as described above and reverse-transcribed by using iScript™ Reverse Transcription Supermix (Bio-Rad, Hercules, CA, USA), according to the manufacturer's protocol.
Then, the expression of HIF-1α gene was evaluated by qRT-PCR as described in Supplementary methods.
HIF-1α activityNuclear extract lysates were obtained from CaCo2 cells by using a Nuclear Extraction Kit (ab221978, Abcam). The protein concentrations of nuclear fractions were determined by Bradford assay using CLARIOstar® Plus plate reader (BMG LABTECH, Germany).
The activities of HIF-1α in nuclear extract lysates were detected using the HIF-1α Transcription Factor Assay Kit (ab133104, Abcam) according to the manufacturer's protocol.
Statistical analysisComparisons between groups were performed by ANOVA or Kruskal–Wallis test depending on whether the data were normally distributed or not. The data are presented as mean±standard deviations (SD) or median±range, depending on the normality of values.
Results were considered significant when p values were ≤0.05.
All the statistical analyses were performed using GraphPad Prism software (version 9.0, GraphPad Software).
Spearman's correlation was used to assess relationships between miRNAs expression levels and main nocturnal respiratory data in OSA and ONCO-OSA patients. Cluster analysis was performed on miRNAs’ expression value in order to identify their relationships. A p-value below 0.05 has been considered statistically significant. GraphPad Software (version 9.0, GraphPad Software) and Orange (version 3.0, University of Ljubljana, Slovenia) were used for the analysis.
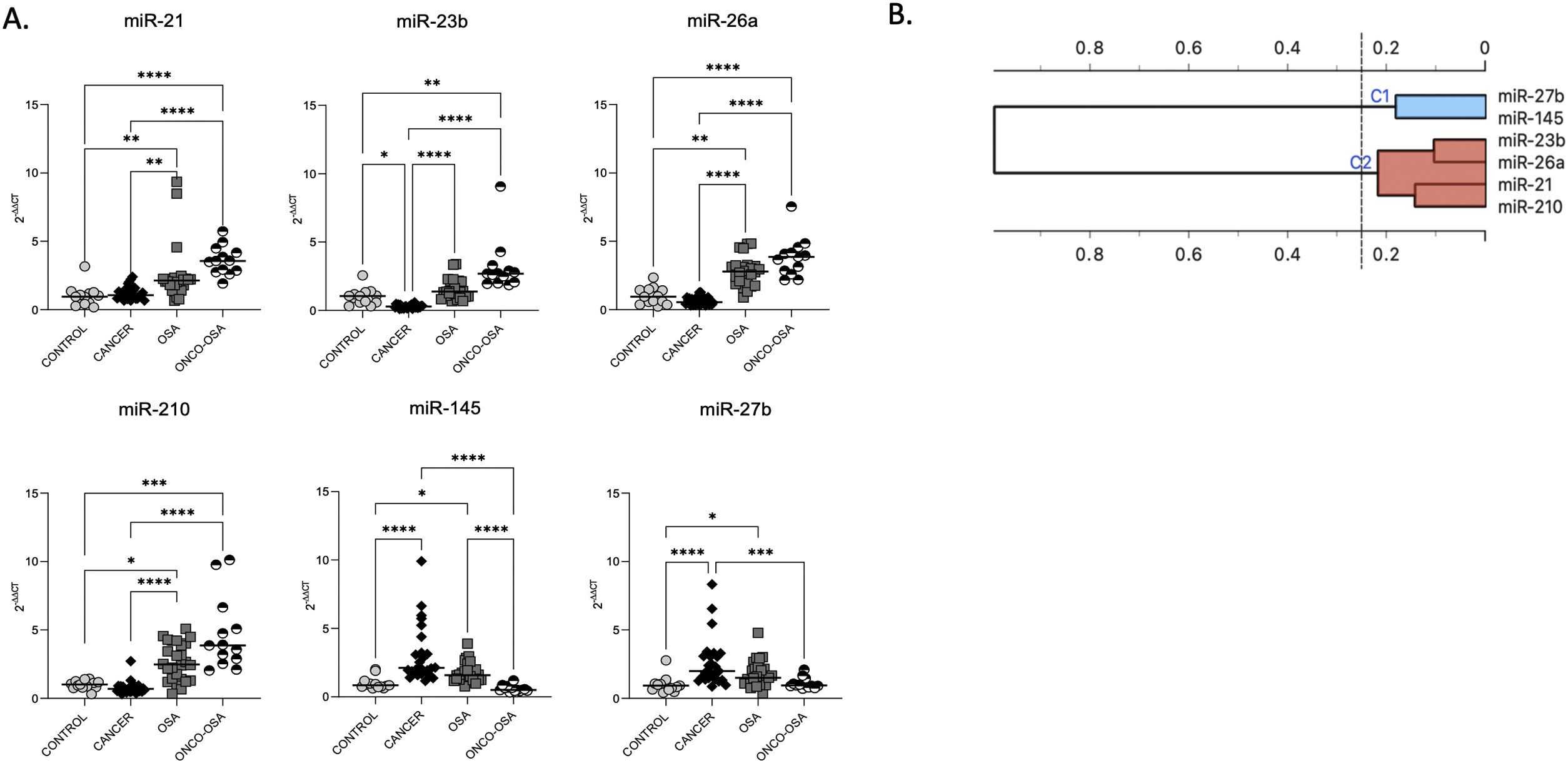
ResultsOSA and cancer differentially regulate miRNA levelsThe expression of miR-21, miR-26a and miR-210 was significantly higher in OSA and in ONCO-OSA patients compared to control and cancer non OSA patients (Fig. 1). miR-23b instead, was significantly lower in cancer patients than in controls, and higher in ONCO-OSA than in cancer and control patients.
miRNA expression in patients. (A) miRNA's expression in patients. Quantitative real-time PCR analysis of differentially expressed microRNAs in healthy subjects compared to patients with cancer, OSA and both cancer and OSA (ONCO-OSA). RNU-6B was used as endogenous control. Comparisons between groups were performed by Kruskal–Wallis test. Individual data and median are plotted. *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001. (B) Clustering of miRNAs. The results from the four patients’ groups were considered together for this analysis. The strength of correlation is inversely indicated by black line's length. Ward's method was applied in cluster analysis. Height ratio: 25% is shown.
miR-27b and miR-145 were significantly increased in both cancer and OSA groups compared to controls, but expression was reduced in ONCO-OSA patients compared to cancer patients (Fig. 1A).
Cluster analysis showed that miR-21, miR-23b, miR-26a and miR-210 had similar behavior (Fig. 1B).
The correlation between the level of miRNAs and indices of hypoxia severity are reported in Supplementary Table S3. SpO2 correlated negatively with miR-21, miR-23, miR-26a and miR-210, and positively with miR-27b and miR-145. AHI and ODI correlated positively with miR-21, miR-23, miR-26a and miR-210, and negatively with miR-145. Finally, TS90 correlated positively with miR-21 and miR-23.
Considering that the majority of our patients (23/39) had colorectal cancer, we subdivided them further considering only oncology patients with CRC. Supplementary Fig. S1A shows results considering only CRC patients. In addition, we assessed the difference of miRNA expression in CRC patients versus all other cancer types (Supplementary Fig. S1B).
Considering only CRC patients, the total number was smaller, but the results are more or less the same as using all patients (Supplementary Fig. S1A). However, making a comparison between CRC versus other types of cancer in cancer and ONCO-OSA patients, no significant differences were found, except for miR-21 which showed a difference in the cancer group between CRC and others (Supplementary Fig. S1B). This suggests that the miRNA variations are globally robustly found in all patients, and are not depending on the cancer type.
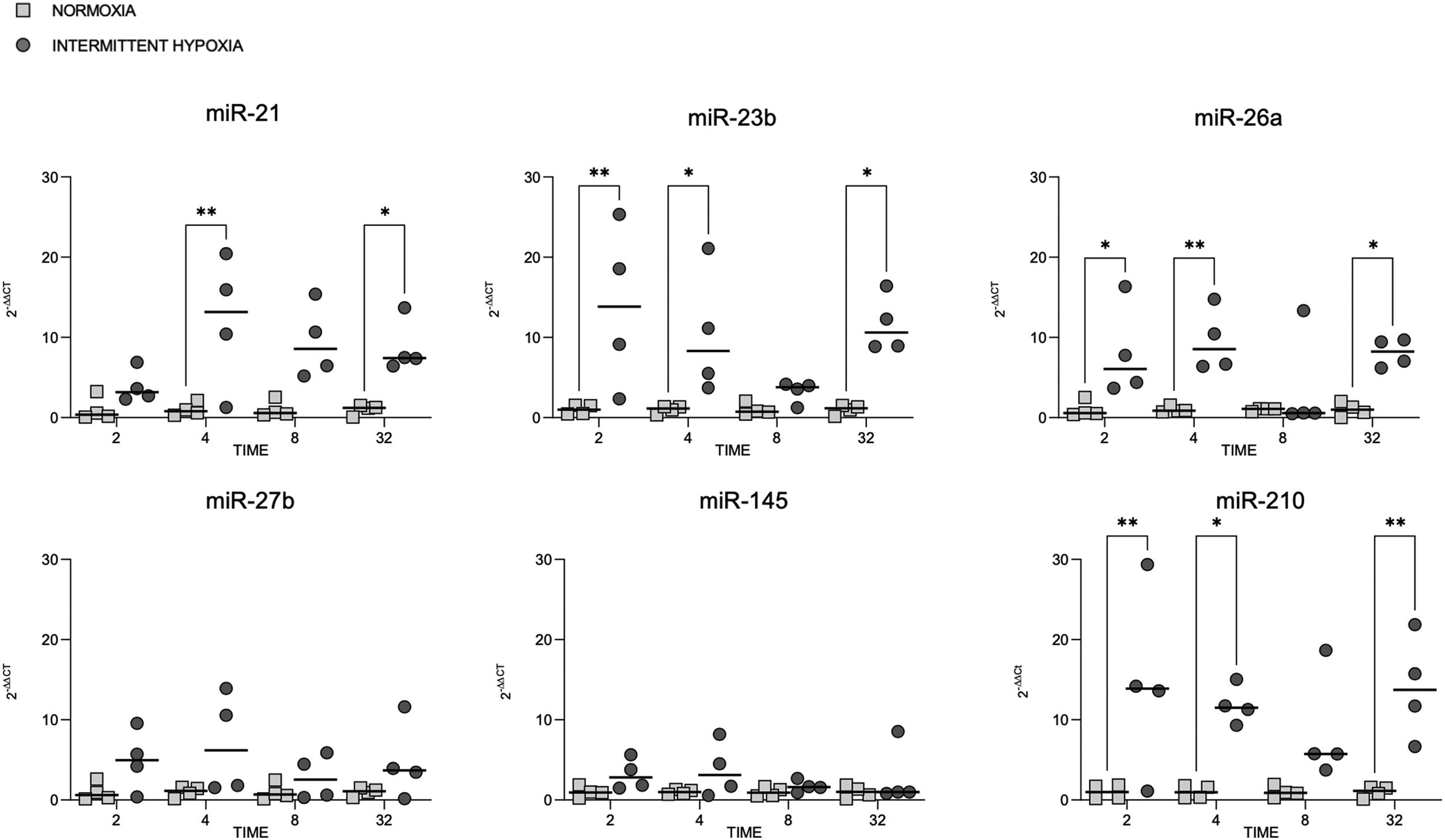
microRNA expression increases in colorectal cancer cells submitted to IHMiRNAs expression was also tested in colorectal cancer cell line (CaCo2) subjected to normoxia versus intermittent hypoxia for 2, 4, 8 and 32h.
The expression of miR-23b, miR-26a and miR-210 at 2, 4 and 32h was highest after IH exposure (Fig. 2). MiR-21 increased in cells exposed to IH for 4 and 32h compared to normoxia. MiR-27b and miR-145 showed no significant variation, whatever the exposure time tested (Fig. 2).
Cellular expression of different microRNAs in normoxia and IH. Quantitative real-time PCR analysis of differentially expressed microRNAs in CaCo2 cells in normoxia vs intermittent hypoxia. Comparisons between groups were performed by two-way ANOVA. Individual data and median are plotted (n=4). *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001.
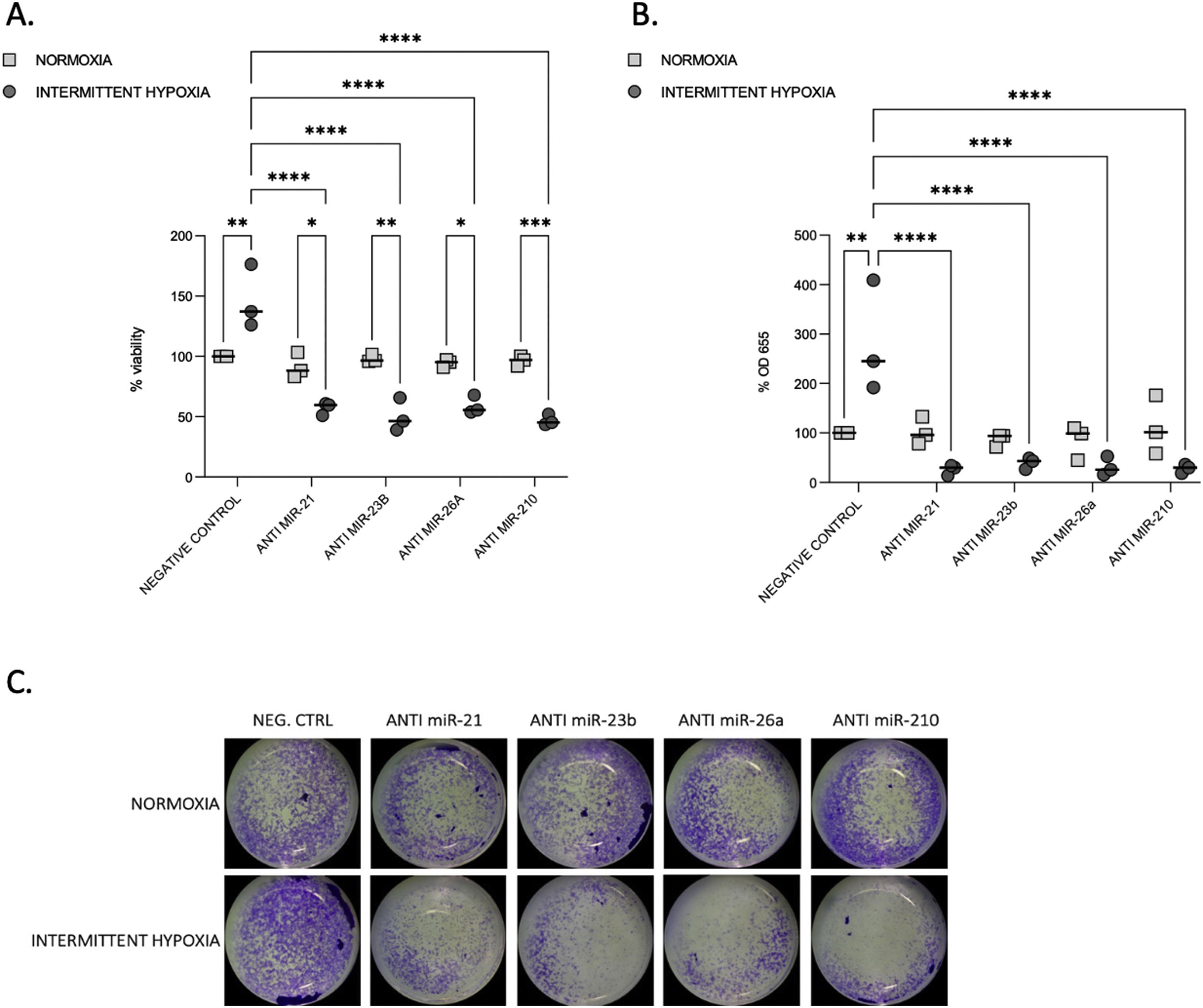
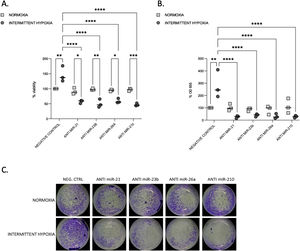
We tested the impact of IH on cell proliferation and migration. We observed that after 32–48h, IH stimulated both cell proliferation and migration (Fig. 3A–C).
Proliferation and migration test in CaCo2 cells both in normoxia and IH after miRNA inhibition. (A) Proliferation test. CaCo2 cells were transfected with various miRNA inhibitor and then incubated in normoxia or IH for 32h. Cells were then assayed for viability by the MTT assay. Cells in normoxia transfected with negative control represented 100% of viability. Individual data and median are plotted (n=3). *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001. (B) Transwell migration assay. Transwell experiments were performed to analyze the cell migration and invasion in CaCo2 cancer cells exposed to normoxia or intermittent hypoxia for 48h and transfected with miRNA inhibitors. Individual data and median are plotted (n=3). *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001. (C) Pictures of the lower side of transwells after migration test and crystal violet staining, taken under a light microscope coupled to a camera at ×10 magnification.
To investigate the functional role of miRNAs, we used transient transfection of antisense inhibitors of miR-21, miR-23b, miR-26a and miR-210, to silence their expression in CaCo2 cells. We confirmed that all these four miRNA expressions can be significantly suppressed by their respective inhibitors (Supplementary Fig. S2). Inhibition of each microRNA reduced proliferation (Fig. 3A) and migration (Fig. 3B and C) compared to IH negative control (p≤0.0001) and to normoxia.
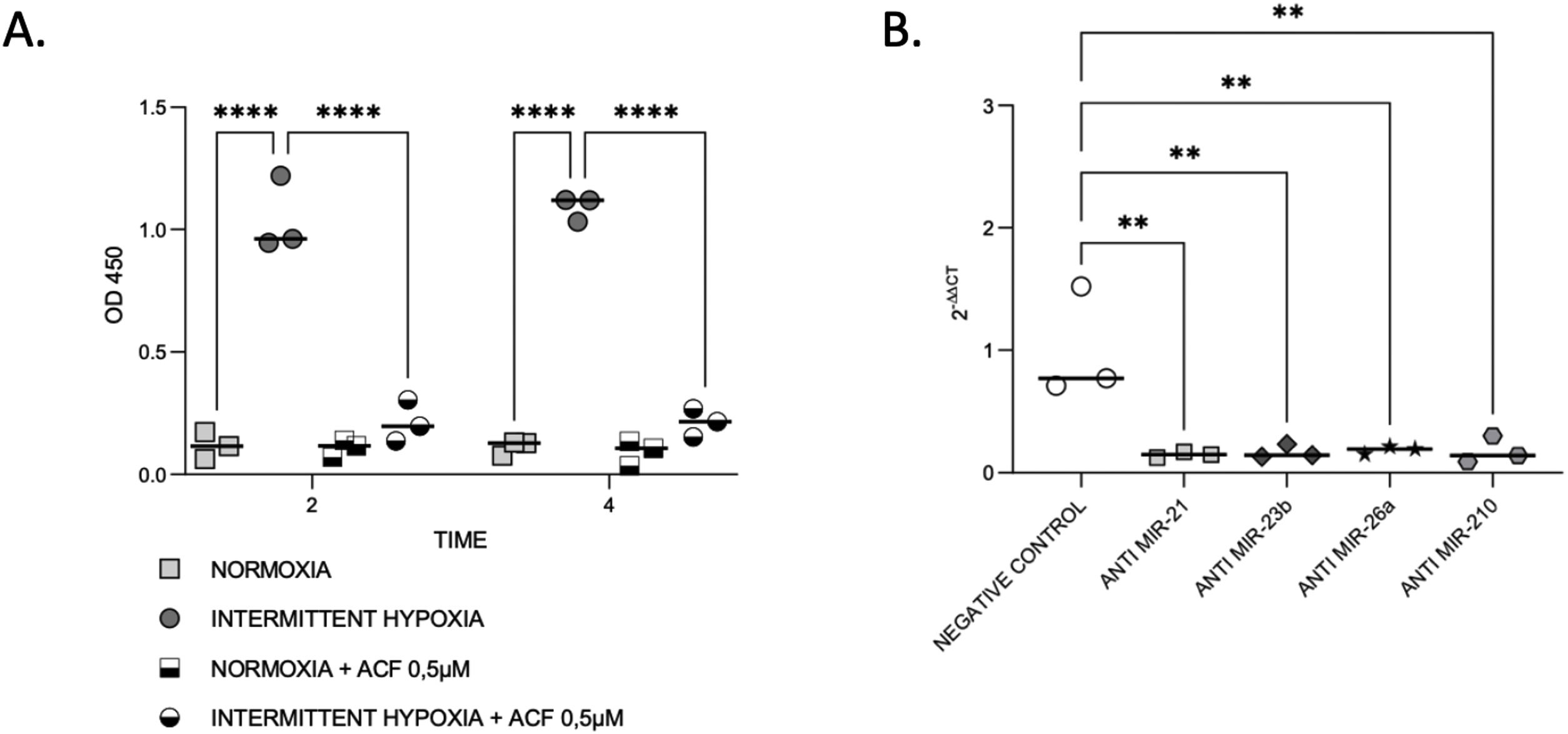
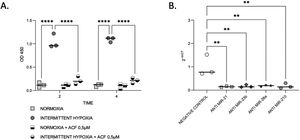
HIF-1α expression is reduced by microRNA inhibitionThen, we aimed to evaluate the links between IH, HIF-1 and miRNA. First, we observed that HIF-1α activity was increased by up to 9-fold after 4h of IH exposure (p≤0.0001). As expected, this HIF-1 activity was abolished after treatment with 0.5μM ACF (p≤0.0001) (Fig. 4A).
IH and miRNAs regulate HIF-1. (A) Treatment with ACF abolished IH-induced HIF-1α activity. Measurement of HIF-1α activity in CaCo2 cells exposed to normoxia or intermittent hypoxia for 2 and 4h, in presence or absence of 0.5μM ACF. Individual data and median are plotted (n=3). ****p≤0.0001. (B) MiRNA inhibition suppresses HIF-1α gene expression. qRT-PCR of HIF-1 gene expression in CaCo2 cells after transfection with miRNA inhibitors. Comparisons between groups were performed by Kruskal–Wallis test. Individual data and median are plotted (n=3). *p≤0.05, **p≤0.01.
Then, HIF-1α gene expression was tested following miRNA inhibition. HIF-1α gene expression was 2–3-fold lower with all inhibitors used (Fig. 4B), suggesting that miRNAs are involved in the regulation of HIF-1α gene expression.
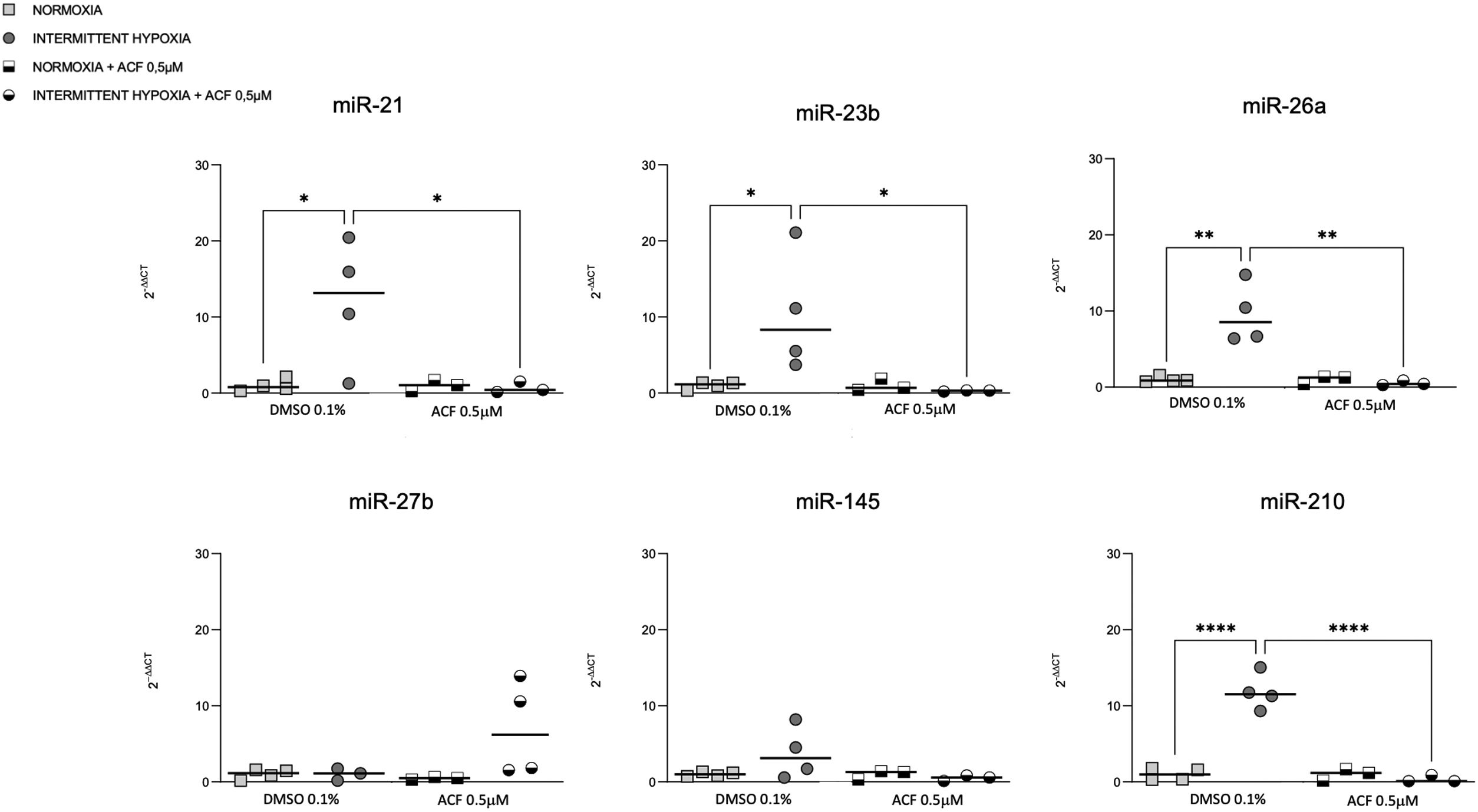
HIF-1 inhibition reverses IH-induced miRNA expressionOn the other hand, we tested if HIF-1 is mediating the regulation of miRNAs by IH. The increase of miR-21, miR-23b, miR-26a and miR-210 expression in CaCo2 after 4h of IH was significantly reversed following 0.5μM ACF treatment (Fig. 5). Similar effects were observed at other time points (2, 8 and 32h) (Supplementary Fig. S3). No differences were found between normoxic conditions with and without ACF. MiR-27b and miR-145 expression did not show any significant difference following treatment with ACF.
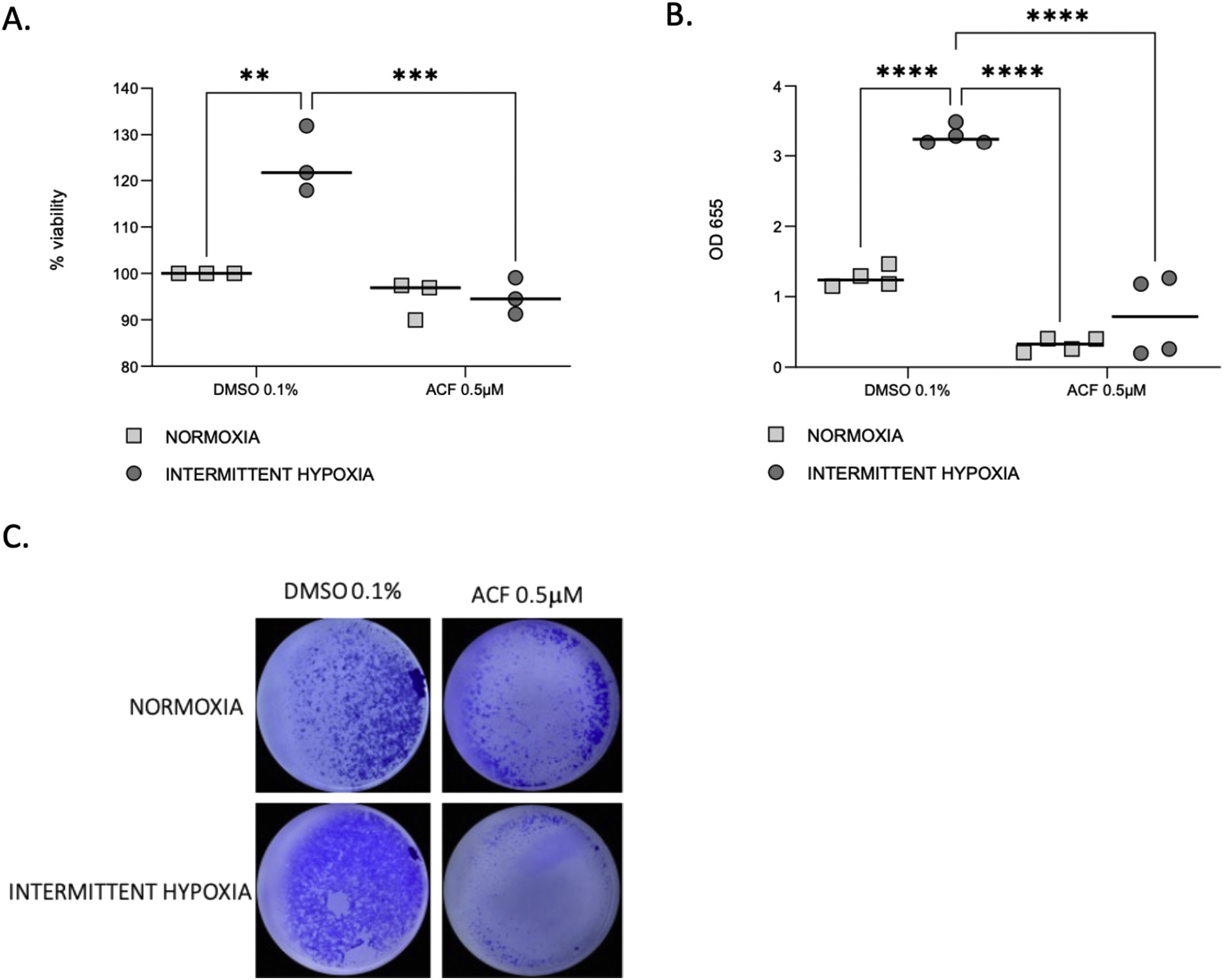
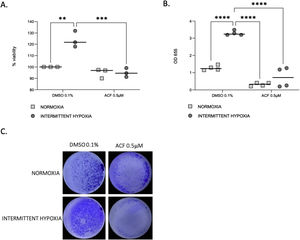
HIF-1 inhibition reverses IH-induced proliferation and migration of CaCo2 cellsProliferation (Fig. 6A) and migration (Fig. 6B and C) of CaCo2 cells were significantly increased in IH in cells treated with DMSO 0.1% as a control (p=0.0014 and p≤0.0001). This increase of proliferation and migration was abolished by 0.5μM ACF (p=0.0004 and p≤0.0001).
ACF reverses IH-induced proliferation and migration of CaCo2 cells. (A) Viability of CaCo2 cells assessed by MTT assay in condition of normoxia and intermittent hypoxia with 0.1% DMSO or 0.5μM of acriflavine (ACF) for 32h. Cells in normoxia treated with DMSO 0.1% represented 100% of viability. Individual data and median are plotted (n=3). **p<0.005. (B) Transwell migration assay were performed to analyze the cell migration after exposure to normoxia or intermittent hypoxia for 48h, in presence or in absence of 0.5μM ACF. Individual data and median are plotted (n=4). **p<0.005. (C) Pictures of the lower side of transwells after migration test and crystal violet staining, taken under a light microscope coupled to a camera at ×10 magnification.
To our best knowledge, this is the first translational study to combine microRNA analyses in healthy individuals, OSA patients with or without cancer and in vitro approaches to explore the effects of IH exposure mimicking OSA on colorectal cancer development and progression. We found significant differences in miRNA expression in OSA and ONCO-OSA groups. We also demonstrated that in vitro, intermittent hypoxia induces increased cell proliferation and migration and these effects were mediated by the same microRNAs.
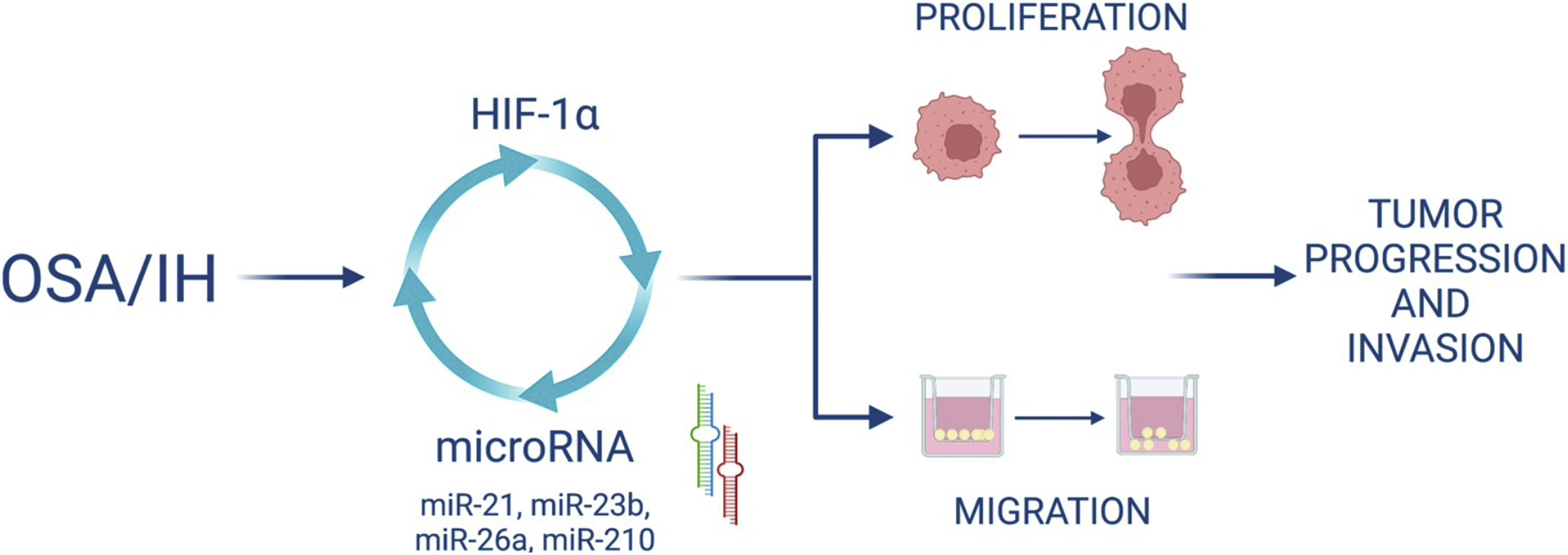
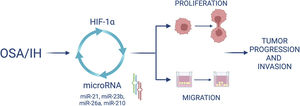
Furthermore, we demonstrated that HIF-1α activity increased after IH and that blocking HIF-1 with acriflavine abolished the IH-induced increase in miRNA expression and cell proliferation and migration. This suggests that HIF-1 mediates cell proliferation and migration partly through miRNA upregulation. Finally, HIF-1 gene expression was decreased following the inhibition of some miRNAs, suggesting a feedback loop by which miRNAs can in turn regulate HIF-1 expression (Fig. 7).
The results of this study elucidated that miR-21, miR-23b, miR-26a and miR-210 expressions were elevated in ONCO-OSA patients compared to non-OSA cancer patients and healthy controls. The same miRNAs were up-regulated in CaCo2 cells following IH exposure. In addition, miR-21, miR-26a and miR-210 had a higher expression in OSA compared to cancer and healthy controls. miRNAs expression correlated with the severity of sleep apnoea as evaluated by AHI, ODI and SpO2.
Clustering analysis allows the identification of miRNAs with similar expression profiles, that are generally involved in the same cellular functions or the same regulatory pathways.23 We showed that miR-21, miR-23b, miR-26a and miR-210 clustered together, while miR-27b and miR-145 were in a different cluster. These results are consistent with the fact that the 4 first miRNAs positively correlate with sleep parameters (AHI, ODI, SpO2) while the other 2 negatively correlate with the same parameters. It was also consistent with their regulation in CaCo2 cells, and with the finding that inhibiting miR-210, miR-21, miR-23b or miR-26a led to the reversion of IH-induced cell proliferation and migration.
It is known that miR-210 mediates important processes associated with tumorigenesis such as proliferation or angiogenesis. In fact, it targets many other miRNAs encoding proteins that play key roles in proliferation, DNA and RNA binding and repair, differentiation, development and apoptosis.24,25 miR-210 has been reported as involved in colorectal cancer migration and invasion through HIF-1.18 Our results about miR-210 expression agree with previous studies showing an upregulation of miR-210 in OSA patients.10
miR-21 and miR-23b have been described as up-regulated in many tumors15,26,27 and were elevated in one study investigating their expression in OSA patients.9 miR-21 was up-regulated in mice models of OSA28,29 but it was not consistent with another study reporting that miR-21 and miR-23 expression was down-regulated in OSA patients.30
MiR-26a is induced by hypoxia and its expression is up-regulated during cell differentiation. Previous studies have demonstrated its involvement in different types of cancers, and its target genes are involved in cellular process such as proliferation, differentiation, apoptosis, invasion and metastasis.16 miR-26a was associated with OSA severity in patients,31 although conflicting data showed that miR-26a was up-regulated by sustained but not intermittent hypoxia.10
Interestingly, although these 4 miRNAs are usually associated with cancer, in our cohort they were not elevated in cancer patients without OSA. By contrast, they were elevated in OSA patients (except for miR-23) and in ONCO-OSA patients, suggesting that OSA rather than cancer is responsible for the serum expression of these miRNAs.
On the other hand, miR-145 and miR-27b are generally down-regulated in various types of cancer and considered as inhibitors of tumor cell proliferation and migration,17,32,33 although conflicting data showed that miR-27 stimulated cell proliferation and invasion.34 In our study, these 2 miRNAs were found elevated in cancer patients and OSA patients but not in ONCO-OSA patients and in CaCo2 cells exposed to IH, suggesting a specific regulatory mechanism when OSA and cancer are combined.
Interconnexion of HIF-1 and miRNA expressionIn this study, we wanted to investigate the involvement of HIF-1 in the expression of specific miRNAs and its role in proliferation and migration of cancer cells exposed to intermittent hypoxia. In this regard, we used acriflavine, an inhibitor of HIF-1 dimerization, which decreased HIF-1 transcriptional activity and showed anticancer efficacy in vivo.35 We showed that inhibiting HIF-1 with acriflavine abolished the IH-induced increase in miRNA expression. This suggests that HIF-1 is necessary for miRNA upregulation. This is consistent with data showing that miR-210,18 miR-21,36 miR-26a16 are targets of HIF-1. On the other hand, we observed that inhibition of miR-21, miR-23b, miR-26a and miR-210 in turn led to significant reduction of HIF-1α gene expression. HIF-1 has indeed been described as a target of miR-21,14 miR-23b15 and miR-26a.37 Our data thus support the hypothesis of a hypoxia-triggered feedback loop involving the expression of HIF-1 and several miRNAs (Fig. 7).
We showed that acriflavine inhibited proliferation and migration of colorectal cancer cell exposed to IH acriflavine treatment. This is line with existing data showing the regulatory role of acriflavine in tumors35,38 and with the known role of HIF-1 in tumor progression induced by IH.39 Altogether, our results highlight a loop between HIF-1 and some miRNAs induced by IH/OSA and contributing to cancer development and progression. This hypothesis is recapitulated in Fig. 7.
Limitations of the studyDespite the relatively small population analyzed, we evidenced statistically significant differences in miRNA expression, suggesting that the variations are strong enough to be detected in this small cohort. Certainly, further studies on a larger and more carefully matched population will be needed to confirm our results.
Moreover, due to the predominance of colorectal cancer in the cancer and ONCO-OSA groups, we chose colorectal cancer cells (CaCo2) to investigate the cellular mechanisms induced by IH. Further studies will be required to confirm these mechanisms in other colorectal cancer cell lines and other types of cancer.
ConclusionThis study found an abundance of differentially expressed miRNAs both in patients affected by OSA, cancer and OSA plus cancer and in colorectal cancer cell exposed to IH. To our knowledge, this is the first time that expression of miRNAs was examined at the clinical and preclinical level and that their functional consequences on IH-induced tumor progression are demonstrated. We postulate that those miRNAs might play a pivotal role in the mechanism of OSA aggravating tumor development and progression.
Moreover, the present study confirms the fundamental role of HIF-1 as a master regulator of tumor cells response to hypoxia, and brings to light a loop between OSA-associated intermittent hypoxia, HIF-1 and microRNA that needs to be further investigated but which could provide some initial insights into the mechanism linking OSA and cancer.
Further studies are warranted to confirm our observations and investigate the specific signaling pathways of each microRNA. As suggested by previous studies,40 targeting HIF-1 and/or the miRNA could represent new therapeutic strategies in OSA patients affected by cancer.
Authors’ contributionsConceptualization, G.M., A.B.M., and D.L.; methodology, G.M., M.M., A.B.M., and D.L.; formal analysis, G.M., A.B.M., P.T., and D.L.; resources, G.M., P.S., M.M., G.S., and A.B.M.; data curation, G.M., A.B.M., and D.L.; writing—original draft preparation, G.M. and A.B.M.; writing—review and editing, all authors; supervision, J.L.P., M.P.F.B., A.B.M., and D.L.; project administration, J.L.P., M.P.F.B., A.B.M., and D.L.
All authors have read and agreed to the published version of the manuscript.
Institutional review board statementThe study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Policlinico Riuniti of Foggia (approval number 17/CE/2014) and Comité de Protection des Personnes Sud-Est V, Grenoble, France.
Informed consent statementInformed consent was obtained from all subjects involved in the study.
Data availability statementNot applicable.
FundingThis research was funded by University of Foggia, University Grenoble Alpes, INSERM, Agir Pour les Maladies Chroniques foundation.
Conflict of interestsThe authors declare no conflict of interest.