Cytochrome P450 (CYP) 2J2 is a major enzyme that controls epoxyeicosatrienoic acids biosynthesis, which may play a role in chronic obstructive pulmonary disease (COPD) development. In this study, we aimed to assess the influence of CYP2J2 polymorphisms with COPD susceptibility.
Material and methodsA case–control study enrolled 313 COPD cases and 508 controls was to investigate the association between CYP2J2 polymorphisms and COPD risk. Agena MassARRAY platform was used to genotype CYP2J2 polymorphisms. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to evaluate the association between CYP2J2 polymorphisms and COPD risk.
ResultsWe observed rs11207535 (homozygote: OR=0.08, 95%CI=0.01–0.96, p=0.047; recessive: OR=0.08, 95%CI=0.01–0.94, p=0.044), rs10889159 (homozygote: OR=0.08, 95%CI=0.01–0.92, p=0.043; recessive: OR=0.08, 95%CI=0.01–0.90, p=0.040) and rs1155002 (heterozygote: OR=1.63, 95%CI=1.13–2.36, p=0.009; dominant: OR=1.64, 95%CI=1.15–2.35, p=0.006; additive: OR=1.45, 95%CI=1.09–1.92, p=0.011) were significantly associated with COPD risk. Allelic tests showed T allele of rs2280274 was related to a decreased risk of COPD and T allele of rs1155002 was associated with an increased COPD risk. Stratified analyses indicated the effects of CYP2J2 polymorphisms and COPD risk were dependent on gender and smoking status (p<0.05). Additionally, two haplotypes (Ars11207535Crs10889159Trs1155002 and Ars11207535Crs10889159Crs1155002) significantly decreased COPD risk.
ConclusionIt suggested CYP2J2 polymorphisms were associated with COPD susceptibility in the Chinese Han population.
El citocromo P450 (CYP) 2J2 es una enzima importante que controla la biosíntesis de los ácidos epoxieicosatrienoicos, y que podría desempeñar un papel en el desarrollo de la enfermedad pulmonar obstructiva crónica (EPOC). En este estudio, nuestro objetivo fue evaluar la influencia de los polimorfismos de CYP2J2 en la susceptibilidad a la EPOC.
Materiales y métodosSe realizó un estudio de casos y controles que incluyó 313 casos de EPOC y 508 controles para investigar la asociación entre los polimorfismos de CYP2J2 y el riesgo de desarrollar EPOC. Se utilizó la plataforma Agena MassARRAY para genotipar los polimorfismos de CYP2J2. Se calcularon los odds ratio (OR) con unos intervalos de confianza (IC) del 95% para valorar la asociación entre los polimorfismos de CYP2J2 y el riesgo de la EPOC.
ResultadosObservamos que rs11207535 (homocigoto: OR: 0,08, IC 95%: 0,01-0,96; p=0,047; recesivo: OR: 0,08; IC 95%: 0,01-0,94; p=0,044), rs10889159 (homocigoto: OR: 0,08, IC 95%: 0,01-0,92; p=0,043; recesivo: OR: 0,08, IC 95%: 0,01-0,90; p=0,040) y rs1155002 (heterocigoto: OR: 1,63, IC 95%: 1,13-2,36; p=0,009; dominante: OR: 1,64, IC 95%: 1,15-2,35; p=0,006; aditivo: OR: 1,45, IC 95%: 1,09-1,92; p=0,011) se asociaron significativamente con el riesgo de EPOC. Las pruebas alélicas mostraron que el alelo T de rs2280274 estaba relacionado con una disminución del riesgo de EPOC y el alelo T de rs1155002 se asoció con un mayor riesgo de EPOC. Los análisis estratificados indicaron que los efectos de los polimorfismos de CYP2J2 y el riesgo de EPOC dependían del sexo y del tabaquismo (p<0,05). Además, 2 haplotipos (Ars11207535Crs10889159Trs1155002 y Ars11207535Crs10889159Crs1155002) reducían significativamente el riesgo de la EPOC.
ConclusiónEl estudio sugirió que los polimorfismos de CYP2J2 se asociaban con la susceptibilidad a la EPOC en la población Han China.
Chronic obstructive pulmonary disease (COPD) is the leading cause of morbidity and mortality worldwide, and the prevalence of COPD is rising in China.1,2 COPD is characterized by persistent respiratory symptoms and reversible airflow limitation.3 The development of COPD is caused by the interactions of environmental and genetic factors.4 Cigarette smoking has long been considered a significant risk factor of COPD. Recently, an increasing number of studies proved that genetic characteristics play a vital role in the susceptibility to COPD.5
Cytochrome P450 (CYP) 2J2 gene belongs to the CYP superfamily, which metabolizes arachidonic acid to four regioisomeric epoxyeicosatrienoic acids (5,6-, 8,9-, 11,12-, and 14,15-EET).6 CYP2J2 enzyme has influence on metabolism of endogenous and exogenous compounds. CYP2J2 is the major CYP expressed in heart, placenta, lung, kidney and gastrointestinal tissues.7 Moreover, CYP2J2 is responsible for the drug metabolizing.8 In previous studies, CYP2J2 had been reported to exert biological effects in the cardiovascular system due to its role in endobiotic metabolism.9 Notably, CYP2J2 is found to be overexpressed in various cancers.10 Furthermore, several CYP2J2 polymorphisms have been reported to be associated with susceptibility to multiple diseases.11,12 G-50T is a common single nucleotide polymorphisms (SNP) of CYP2J2, which leads to a decrease in the gene expression and results in an altered epoxygenase-dependent arachidonic acid metabolism of eicosanoids that possess important biological functions in the lung and airways.13 Among the candidate SNPs in our study, rs1155002 was the most popular polymorphism. Studies revealed that rs1155002 was associated with increased risk of late-onset Alzheimer's disease and hypertension.14,15 However, a limited number of studies have been conducted to investigate the contributions of CYP2J2 polymorphisms to COPD risk.16–18
Thus, it is necessary to estimate the relationships of COPD risk and CYP2J2 polymorphisms. The present study was designed to explore whether CYP2J2 polymorphisms (rs2280274, rs4388726, rs11207535, rs10889159, and rs1155002) had relationship with the susceptibility of COPD based on a case–control study.
Materials and methodsStudy participantsA total of 313 COPD patients (238 men and 75 women) and 508 healthy controls (337 men and 171 women) were consecutively recruited from Hainan General Hospital. All COPD patients had airway obstruction on the spirometry and clinical features of COPD (cough, sputum production, dyspnea), and they were confirmed as post-bronchodilator FEV1/FVC<70% according to the criteria established by the National Heart, Lung and Blood Institute/World Health Organization Global Initiative for Chronic Obstructive Lung Disease.19 Patients with other respiratory diseases, autoimmune diseases or cancers were excluded from the study. Healthy controls with FEV1/FVC>70% were randomly recruited from the same hospital with patients during the same period, which included the subjects without diseases or family history of any diseases. Informed consents were obtained from all participants. And, our study protocol was strictly conformed to the Declaration of Helsinki and was carried out with the approval from the ethics committee of Hainan General Hospital.
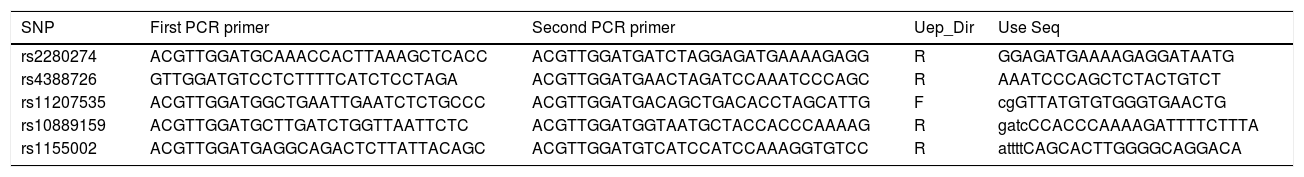
SNP selection and genotypingCombined previous studies, five SNPs of CYP2J2 had minor allele frequencies (MAFs) >5% in the HapMap Chinese Han Beijing population were selected in this study. Genomic DNA was isolated from whole-blood samples using the blood DNA kit (GoldMag Co. Ltd., Xi’an, China), and DNA concentrations were determined by Nanodrop 2000 (Thermo Scientific, Waltham, MA, USA).20 Agena MassARRAY Assay Design 3.0 software was performed to design primers used in the study (Table 1). Genotyping was conducted by Agena MassARRAY system (Agena, San Diego, CA, USA), according to the manufacturer's protocol. In addition, we performed the data management and analysis using the Agena Typer 4.0 Software (San Diego, CA, USA).21
Primers used for this study.
| SNP | First PCR primer | Second PCR primer | Uep_Dir | Use Seq |
|---|---|---|---|---|
| rs2280274 | ACGTTGGATGCAAACCACTTAAAGCTCACC | ACGTTGGATGATCTAGGAGATGAAAAGAGG | R | GGAGATGAAAAGAGGATAATG |
| rs4388726 | GTTGGATGTCCTCTTTTCATCTCCTAGA | ACGTTGGATGAACTAGATCCAAATCCCAGC | R | AAATCCCAGCTCTACTGTCT |
| rs11207535 | ACGTTGGATGGCTGAATTGAATCTCTGCCC | ACGTTGGATGACAGCTGACACCTAGCATTG | F | cgGTTATGTGTGGGTGAACTG |
| rs10889159 | ACGTTGGATGCTTGATCTGGTTAATTCTC | ACGTTGGATGGTAATGCTACCACCCAAAAG | R | gatcCCACCCAAAAGATTTTCTTTA |
| rs1155002 | ACGTTGGATGAGGCAGACTCTTATTACAGC | ACGTTGGATGTCATCCATCCAAAGGTGTCC | R | attttCAGCACTTGGGGCAGGACA |
Abbreviations: SNP, single nucleotide polymorphism; USE SEQ, unextended mini-sequencing primer.
We used SPSS 22.0 statistical package (SPSS, Chicago, IL, USA) to perform statistical analyses. All continuous data were presented as mean±standard deviation. The chi square test and Student's t-test were used to compare the differences of categorical and continuous variables. Hardy–Weinberg equilibrium (HWE) for each SNP was assessed using Fisher's exact test in control group. For evaluating the impact of candidate SNPs on COPD risk, we used logistic regression analyses with adjustment for age and gender to estimate the odds ratio (OR) and 95% confidence interval (CI). Then, genetic models (co-dominant, dominant, recessive and additive) were used to evaluate the associations between CYP2J2 polymorphisms and COPD risk by PLINK software (version 1.07). Furthermore, we used PLINK software (version 1.07) and Haploview software (version 4.2) for haplotype analysis and linkage disequilibrium (LD).22 All p values shown in our study were two sided, and p<0.05 was regarded as statistically significant.
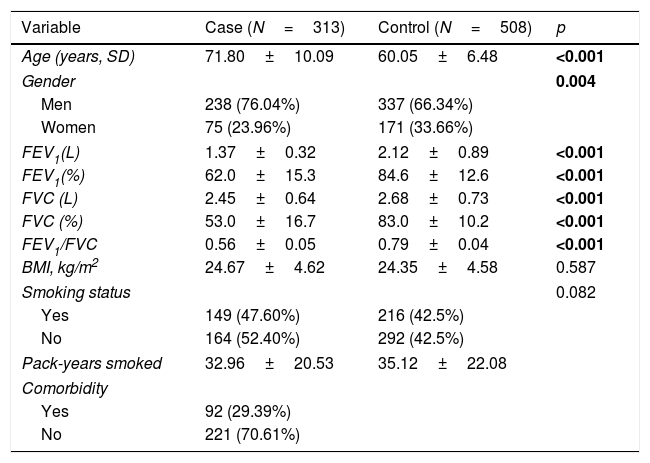
ResultsCharacteristics of the participantsThe basic characteristics of 821 participators in the study are provided in Table 2. The mean ages of cases and controls were 71.80±10.09 and 60.05±6.48 years old. The majority of patients and controls were men (76.04% and 66.34%, respectively). The p values for age and gender were less than 0.05. Compared with the control group, the cases had significantly lower FEV1 (L), FEV1 (%), FVC (L), FVC (%) and FEV1/FVC (p<0.001). There was no significant distribution of BMI in two groups (p=0.587). There are 149 (47.60%) and 216 (42.5%) smokers in cases and controls, individually. The pack-years smoked were 32.96±20.53 and 35.12±22.08. Among the patients with COPD, 92 (29.39%) had comorbidity.
The characteristic of case and control.
| Variable | Case (N=313) | Control (N=508) | p |
|---|---|---|---|
| Age (years, SD) | 71.80±10.09 | 60.05±6.48 | <0.001 |
| Gender | 0.004 | ||
| Men | 238 (76.04%) | 337 (66.34%) | |
| Women | 75 (23.96%) | 171 (33.66%) | |
| FEV1(L) | 1.37±0.32 | 2.12±0.89 | <0.001 |
| FEV1(%) | 62.0±15.3 | 84.6±12.6 | <0.001 |
| FVC (L) | 2.45±0.64 | 2.68±0.73 | <0.001 |
| FVC (%) | 53.0±16.7 | 83.0±10.2 | <0.001 |
| FEV1/FVC | 0.56±0.05 | 0.79±0.04 | <0.001 |
| BMI, kg/m2 | 24.67±4.62 | 24.35±4.58 | 0.587 |
| Smoking status | 0.082 | ||
| Yes | 149 (47.60%) | 216 (42.5%) | |
| No | 164 (52.40%) | 292 (42.5%) | |
| Pack-years smoked | 32.96±20.53 | 35.12±22.08 | |
| Comorbidity | |||
| Yes | 92 (29.39%) | ||
| No | 221 (70.61%) | ||
Abbreviations: SD, standard deviation; FEV1, forced expiratory volume in 1second; FVC, forced volume capacity; BMI, body mass index.
No smoking means never smoked.
p<0.05 indicates statistical significance.
Bold data mean significant difference.
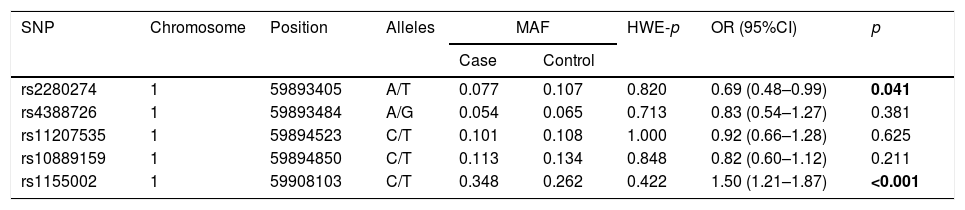
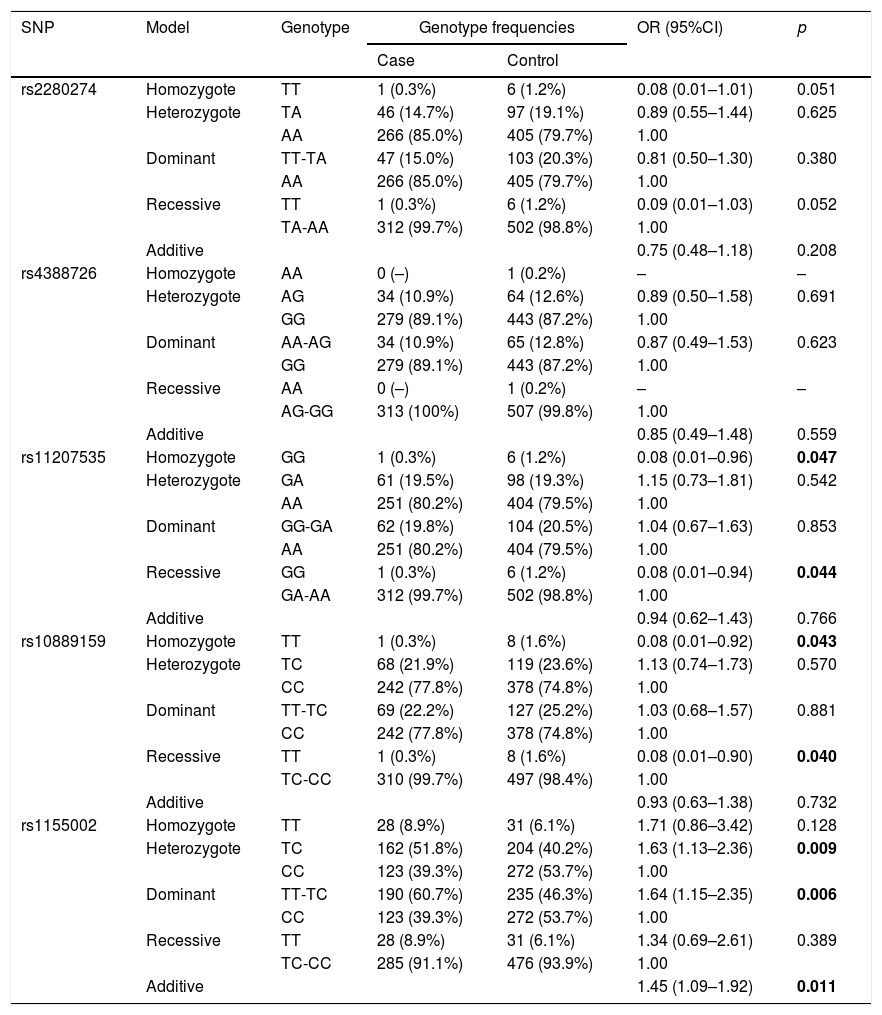
The basic information of the selected SNPs is shown in Table 3. All SNPs were in accord with HWE in the controls (p>0.05). The minor allele frequencies (MAFs) of the analyzed SNPs in the cases and controls were also presented. Then, we compared the differences in frequency distributions of alleles between two groups, and we found rs2280274 and rs1155002 were significantly associated with COPD risk. Among them, the minor allele “T” of rs2280274 decreased the risk of COPD (OR=0.69, 95%CI=0.48–0.99, p=0.041), whereas the individuals carried “T” of rs1155002 were related to higher risk of COPD (OR=1.50, 95%CI=1.21–1.87, p<0.001). After adjustment for age and gender, the genotype frequencies of CYP2J2 polymorphisms and their association with COPD risk in the genetic models are shown in Table 4. We found that GG genotype of rs11207535 was associated with a decreased risk of COPD in the homozygote model (OR=0.08, 95%CI=0.01–0.96, p=0.047) and recessive model (OR=0.08, 95%CI=0.01–0.94, p=0.044). Meanwhile, rs10889159 had a strong relationship with lower COPD risk in homozygote model (OR=0.08, 95%CI=0.01–0.92, p=0.043) and recessive model (OR=0.08, 95%CI=0.01–0.90, p=0.040). In addition, multiple model analyses revealed that rs1155002 was positively associated with higher susceptibility of COPD (heterozygote: OR=1.63, 95%CI=1.13–2.36, p=0.009; dominant: OR=1.64, 95%CI=1.15–2.35, p=0.006; additive: OR=1.45, 95%CI=1.09–1.92, p=0.011).
Basic information of candidate SNPs.
| SNP | Chromosome | Position | Alleles | MAF | HWE-p | OR (95%CI) | p | |
|---|---|---|---|---|---|---|---|---|
| Case | Control | |||||||
| rs2280274 | 1 | 59893405 | A/T | 0.077 | 0.107 | 0.820 | 0.69 (0.48–0.99) | 0.041 |
| rs4388726 | 1 | 59893484 | A/G | 0.054 | 0.065 | 0.713 | 0.83 (0.54–1.27) | 0.381 |
| rs11207535 | 1 | 59894523 | C/T | 0.101 | 0.108 | 1.000 | 0.92 (0.66–1.28) | 0.625 |
| rs10889159 | 1 | 59894850 | C/T | 0.113 | 0.134 | 0.848 | 0.82 (0.60–1.12) | 0.211 |
| rs1155002 | 1 | 59908103 | C/T | 0.348 | 0.262 | 0.422 | 1.50 (1.21–1.87) | <0.001 |
Abbreviations: SNP, single nucleotide polymorphism; MAF, minor allele frequency; HWE, Hardy–Weinberg equilibrium; OR, odds ratio; CI, confidence interval.
p<0.05 indicates statistical significance.
Bold data mean significant difference.
Genotype frequencies of the SNPs and their associations with risk of COPD.
| SNP | Model | Genotype | Genotype frequencies | OR (95%CI) | p | |
|---|---|---|---|---|---|---|
| Case | Control | |||||
| rs2280274 | Homozygote | TT | 1 (0.3%) | 6 (1.2%) | 0.08 (0.01–1.01) | 0.051 |
| Heterozygote | TA | 46 (14.7%) | 97 (19.1%) | 0.89 (0.55–1.44) | 0.625 | |
| AA | 266 (85.0%) | 405 (79.7%) | 1.00 | |||
| Dominant | TT-TA | 47 (15.0%) | 103 (20.3%) | 0.81 (0.50–1.30) | 0.380 | |
| AA | 266 (85.0%) | 405 (79.7%) | 1.00 | |||
| Recessive | TT | 1 (0.3%) | 6 (1.2%) | 0.09 (0.01–1.03) | 0.052 | |
| TA-AA | 312 (99.7%) | 502 (98.8%) | 1.00 | |||
| Additive | 0.75 (0.48–1.18) | 0.208 | ||||
| rs4388726 | Homozygote | AA | 0 (–) | 1 (0.2%) | – | – |
| Heterozygote | AG | 34 (10.9%) | 64 (12.6%) | 0.89 (0.50–1.58) | 0.691 | |
| GG | 279 (89.1%) | 443 (87.2%) | 1.00 | |||
| Dominant | AA-AG | 34 (10.9%) | 65 (12.8%) | 0.87 (0.49–1.53) | 0.623 | |
| GG | 279 (89.1%) | 443 (87.2%) | 1.00 | |||
| Recessive | AA | 0 (–) | 1 (0.2%) | – | – | |
| AG-GG | 313 (100%) | 507 (99.8%) | 1.00 | |||
| Additive | 0.85 (0.49–1.48) | 0.559 | ||||
| rs11207535 | Homozygote | GG | 1 (0.3%) | 6 (1.2%) | 0.08 (0.01–0.96) | 0.047 |
| Heterozygote | GA | 61 (19.5%) | 98 (19.3%) | 1.15 (0.73–1.81) | 0.542 | |
| AA | 251 (80.2%) | 404 (79.5%) | 1.00 | |||
| Dominant | GG-GA | 62 (19.8%) | 104 (20.5%) | 1.04 (0.67–1.63) | 0.853 | |
| AA | 251 (80.2%) | 404 (79.5%) | 1.00 | |||
| Recessive | GG | 1 (0.3%) | 6 (1.2%) | 0.08 (0.01–0.94) | 0.044 | |
| GA-AA | 312 (99.7%) | 502 (98.8%) | 1.00 | |||
| Additive | 0.94 (0.62–1.43) | 0.766 | ||||
| rs10889159 | Homozygote | TT | 1 (0.3%) | 8 (1.6%) | 0.08 (0.01–0.92) | 0.043 |
| Heterozygote | TC | 68 (21.9%) | 119 (23.6%) | 1.13 (0.74–1.73) | 0.570 | |
| CC | 242 (77.8%) | 378 (74.8%) | 1.00 | |||
| Dominant | TT-TC | 69 (22.2%) | 127 (25.2%) | 1.03 (0.68–1.57) | 0.881 | |
| CC | 242 (77.8%) | 378 (74.8%) | 1.00 | |||
| Recessive | TT | 1 (0.3%) | 8 (1.6%) | 0.08 (0.01–0.90) | 0.040 | |
| TC-CC | 310 (99.7%) | 497 (98.4%) | 1.00 | |||
| Additive | 0.93 (0.63–1.38) | 0.732 | ||||
| rs1155002 | Homozygote | TT | 28 (8.9%) | 31 (6.1%) | 1.71 (0.86–3.42) | 0.128 |
| Heterozygote | TC | 162 (51.8%) | 204 (40.2%) | 1.63 (1.13–2.36) | 0.009 | |
| CC | 123 (39.3%) | 272 (53.7%) | 1.00 | |||
| Dominant | TT-TC | 190 (60.7%) | 235 (46.3%) | 1.64 (1.15–2.35) | 0.006 | |
| CC | 123 (39.3%) | 272 (53.7%) | 1.00 | |||
| Recessive | TT | 28 (8.9%) | 31 (6.1%) | 1.34 (0.69–2.61) | 0.389 | |
| TC-CC | 285 (91.1%) | 476 (93.9%) | 1.00 | |||
| Additive | 1.45 (1.09–1.92) | 0.011 | ||||
Abbreviations: SNP, single nucleotide polymorphism; COPD, chronic obstructive pulmonary disease; OR, odds ratio; CI, confidence interval.
p<0.05 indicates statistical significance.
Bold data mean significant difference.
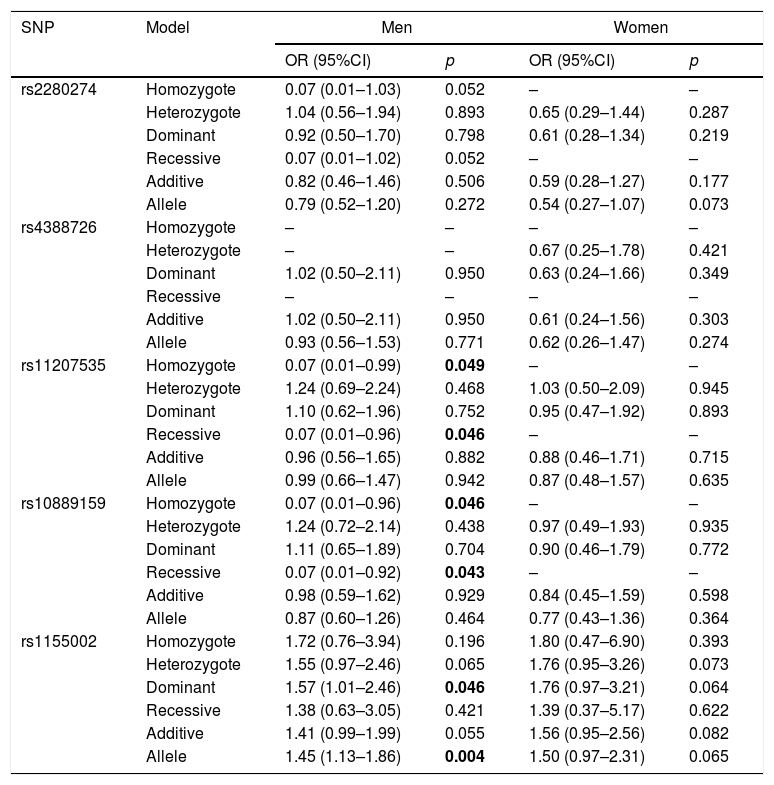
The association between CYP2J2 polymorphisms and COPD risk was further assessed in subgroup of gender (Table 5). For men, rs11207535 and rs10889159 of CYP2J2 were associated with a decreased COPD risk in homozygote (rs11207535: OR=0.07, 95%CI=0.01–0.99, p=0.049; rs10889159: OR=0.07, 95%CI=0.01–0.96, p=0.046) and recessive (rs11207535: OR=0.07, 95%CI=0.01–0.96, p=0.046; rs10889159: OR=0.07, 95%CI=0.01–0.92, p=0.043) models, when compared to the respective reference groups. And, rs1155002 was associated with higher COPD risk in dominant (OR=1.57, 95%CI=1.01–2.46, p=0.046) and allele (OR=1.45, 95%CI=1.13–1.86, p=0.004) models. The other SNPs did not have effects on COPD susceptibility for all participants (p>0.05).
The association between CYP2J2 SNPs and COPD risk stratified by sex.
| SNP | Model | Men | Women | ||
|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | ||
| rs2280274 | Homozygote | 0.07 (0.01–1.03) | 0.052 | – | – |
| Heterozygote | 1.04 (0.56–1.94) | 0.893 | 0.65 (0.29–1.44) | 0.287 | |
| Dominant | 0.92 (0.50–1.70) | 0.798 | 0.61 (0.28–1.34) | 0.219 | |
| Recessive | 0.07 (0.01–1.02) | 0.052 | – | – | |
| Additive | 0.82 (0.46–1.46) | 0.506 | 0.59 (0.28–1.27) | 0.177 | |
| Allele | 0.79 (0.52–1.20) | 0.272 | 0.54 (0.27–1.07) | 0.073 | |
| rs4388726 | Homozygote | – | – | – | – |
| Heterozygote | – | – | 0.67 (0.25–1.78) | 0.421 | |
| Dominant | 1.02 (0.50–2.11) | 0.950 | 0.63 (0.24–1.66) | 0.349 | |
| Recessive | – | – | – | – | |
| Additive | 1.02 (0.50–2.11) | 0.950 | 0.61 (0.24–1.56) | 0.303 | |
| Allele | 0.93 (0.56–1.53) | 0.771 | 0.62 (0.26–1.47) | 0.274 | |
| rs11207535 | Homozygote | 0.07 (0.01–0.99) | 0.049 | – | – |
| Heterozygote | 1.24 (0.69–2.24) | 0.468 | 1.03 (0.50–2.09) | 0.945 | |
| Dominant | 1.10 (0.62–1.96) | 0.752 | 0.95 (0.47–1.92) | 0.893 | |
| Recessive | 0.07 (0.01–0.96) | 0.046 | – | – | |
| Additive | 0.96 (0.56–1.65) | 0.882 | 0.88 (0.46–1.71) | 0.715 | |
| Allele | 0.99 (0.66–1.47) | 0.942 | 0.87 (0.48–1.57) | 0.635 | |
| rs10889159 | Homozygote | 0.07 (0.01–0.96) | 0.046 | – | – |
| Heterozygote | 1.24 (0.72–2.14) | 0.438 | 0.97 (0.49–1.93) | 0.935 | |
| Dominant | 1.11 (0.65–1.89) | 0.704 | 0.90 (0.46–1.79) | 0.772 | |
| Recessive | 0.07 (0.01–0.92) | 0.043 | – | – | |
| Additive | 0.98 (0.59–1.62) | 0.929 | 0.84 (0.45–1.59) | 0.598 | |
| Allele | 0.87 (0.60–1.26) | 0.464 | 0.77 (0.43–1.36) | 0.364 | |
| rs1155002 | Homozygote | 1.72 (0.76–3.94) | 0.196 | 1.80 (0.47–6.90) | 0.393 |
| Heterozygote | 1.55 (0.97–2.46) | 0.065 | 1.76 (0.95–3.26) | 0.073 | |
| Dominant | 1.57 (1.01–2.46) | 0.046 | 1.76 (0.97–3.21) | 0.064 | |
| Recessive | 1.38 (0.63–3.05) | 0.421 | 1.39 (0.37–5.17) | 0.622 | |
| Additive | 1.41 (0.99–1.99) | 0.055 | 1.56 (0.95–2.56) | 0.082 | |
| Allele | 1.45 (1.13–1.86) | 0.004 | 1.50 (0.97–2.31) | 0.065 | |
Abbreviations: SNP, single nucleotide polymorphism; COPD, chronic obstructive pulmonary disease; OR, odds ratio; CI, confidence interval.
p<0.05 indicates statistical significance.
Bold data mean significant difference.
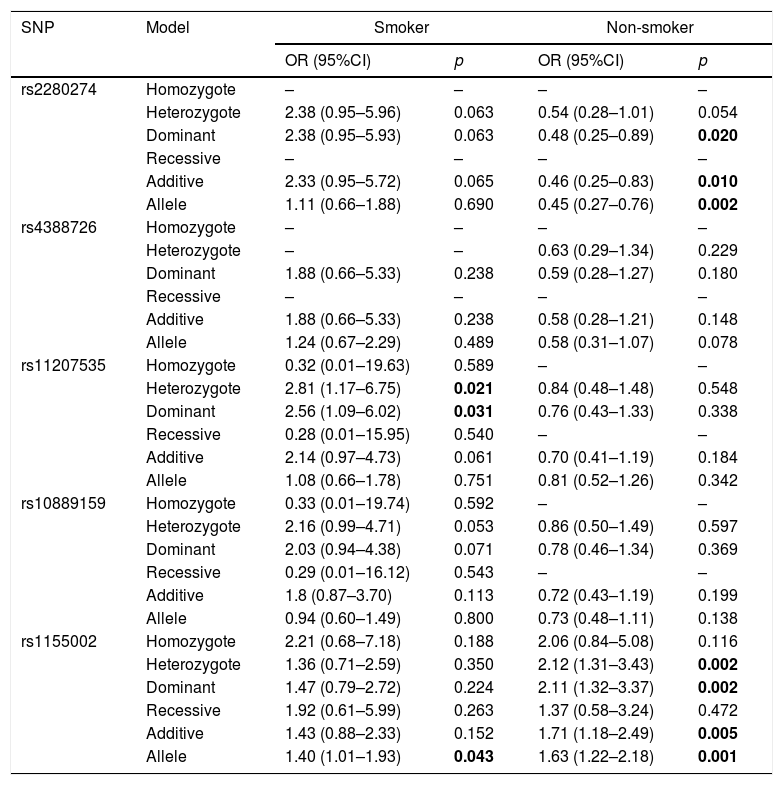
Then, we did the association of CYP2J2 SNPs and COPD risk stratified by smoking status (Table 6). For smoker, rs11207535 and rs1155002 had strong relationship with increased risk of COPD (rs11207535, heterozygote: OR=2.81, 95%CI=1.17–6.75, p=0.021, dominant: OR=2.56, 95%CI=1.09–6.02, p=0.031; rs1155002: OR=1.40, 95%CI=1.01–1.93, p=0.043). Rs2280274 and rs1155002 were associated with COPD risk among non-smoker, rs2280274 significantly decreased COPD risk in dominant (OR=0.48, 95%CI=0.25–0.89, p=0.020), additive (OR=0.46, 95%CI=0.25–0.83, p=0.010) and allele (OR=0.45, 95%CI=0.27–0.76, p=0.002) models, rs1155002 was significantly associated with higher risk of COPD (heterozygote: OR=2.12, 95%CI=1.31–3.43, p=0.002; dominant: OR=2.11, 95%CI=1.32–3.37, p=0.002; additive: OR=1.71, 95%CI=1.18–2.49, p=0.005; allele: OR=1.63, 95%CI=1.22–2.18, p=0.001).
The association between CYP2J2 SNPs and COPD risk stratified by smoking status.
| SNP | Model | Smoker | Non-smoker | ||
|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | ||
| rs2280274 | Homozygote | – | – | – | – |
| Heterozygote | 2.38 (0.95–5.96) | 0.063 | 0.54 (0.28–1.01) | 0.054 | |
| Dominant | 2.38 (0.95–5.93) | 0.063 | 0.48 (0.25–0.89) | 0.020 | |
| Recessive | – | – | – | – | |
| Additive | 2.33 (0.95–5.72) | 0.065 | 0.46 (0.25–0.83) | 0.010 | |
| Allele | 1.11 (0.66–1.88) | 0.690 | 0.45 (0.27–0.76) | 0.002 | |
| rs4388726 | Homozygote | – | – | – | – |
| Heterozygote | – | – | 0.63 (0.29–1.34) | 0.229 | |
| Dominant | 1.88 (0.66–5.33) | 0.238 | 0.59 (0.28–1.27) | 0.180 | |
| Recessive | – | – | – | – | |
| Additive | 1.88 (0.66–5.33) | 0.238 | 0.58 (0.28–1.21) | 0.148 | |
| Allele | 1.24 (0.67–2.29) | 0.489 | 0.58 (0.31–1.07) | 0.078 | |
| rs11207535 | Homozygote | 0.32 (0.01–19.63) | 0.589 | – | – |
| Heterozygote | 2.81 (1.17–6.75) | 0.021 | 0.84 (0.48–1.48) | 0.548 | |
| Dominant | 2.56 (1.09–6.02) | 0.031 | 0.76 (0.43–1.33) | 0.338 | |
| Recessive | 0.28 (0.01–15.95) | 0.540 | – | – | |
| Additive | 2.14 (0.97–4.73) | 0.061 | 0.70 (0.41–1.19) | 0.184 | |
| Allele | 1.08 (0.66–1.78) | 0.751 | 0.81 (0.52–1.26) | 0.342 | |
| rs10889159 | Homozygote | 0.33 (0.01–19.74) | 0.592 | – | – |
| Heterozygote | 2.16 (0.99–4.71) | 0.053 | 0.86 (0.50–1.49) | 0.597 | |
| Dominant | 2.03 (0.94–4.38) | 0.071 | 0.78 (0.46–1.34) | 0.369 | |
| Recessive | 0.29 (0.01–16.12) | 0.543 | – | – | |
| Additive | 1.8 (0.87–3.70) | 0.113 | 0.72 (0.43–1.19) | 0.199 | |
| Allele | 0.94 (0.60–1.49) | 0.800 | 0.73 (0.48–1.11) | 0.138 | |
| rs1155002 | Homozygote | 2.21 (0.68–7.18) | 0.188 | 2.06 (0.84–5.08) | 0.116 |
| Heterozygote | 1.36 (0.71–2.59) | 0.350 | 2.12 (1.31–3.43) | 0.002 | |
| Dominant | 1.47 (0.79–2.72) | 0.224 | 2.11 (1.32–3.37) | 0.002 | |
| Recessive | 1.92 (0.61–5.99) | 0.263 | 1.37 (0.58–3.24) | 0.472 | |
| Additive | 1.43 (0.88–2.33) | 0.152 | 1.71 (1.18–2.49) | 0.005 | |
| Allele | 1.40 (1.01–1.93) | 0.043 | 1.63 (1.22–2.18) | 0.001 | |
Abbreviations: SNP, single nucleotide polymorphism; COPD, chronic obstructive pulmonary disease; OR, odds ratio; CI, confidence interval.
p<0.05 indicates statistical significance.
Bold data mean significant difference.
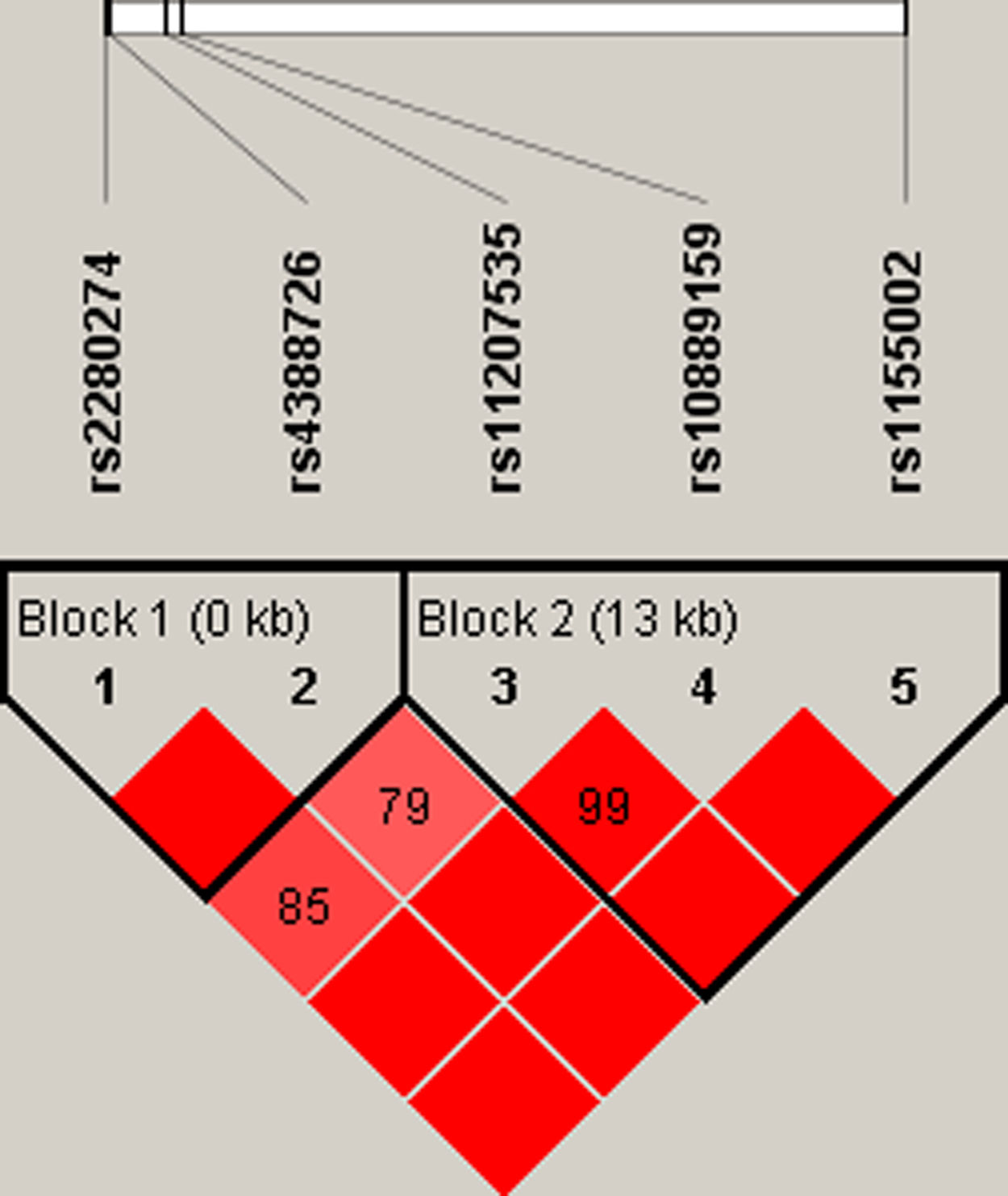
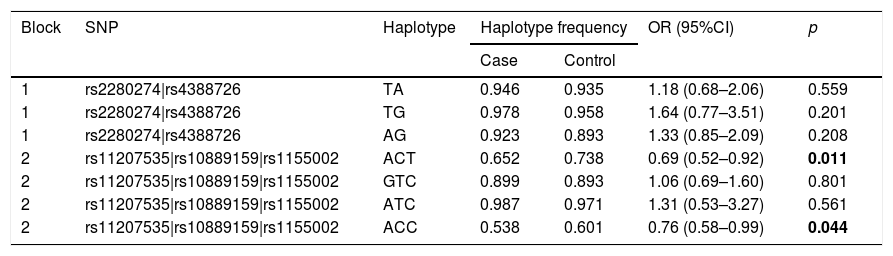
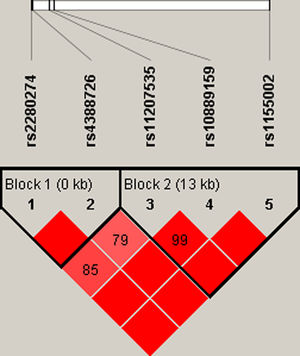
Finally, we did the LD block and found two strong blocks (Fig. 1). The association between haplotypes of CYP2J2 SNPs and COPD risk is listed in Table 7. The “A-C-T” and “A-C-C” haplotypes of rs11207535, rs10889159 and rs1155002 were significantly decreased the COPD risk (OR=0.69, 95%CI=0.52–0.92, p=0.011; OR=0.76, 95%CI=0.58–0.99, p=0.044; respectively).
The haplotype of CYP2J2 SNPs and COPD risk.
| Block | SNP | Haplotype | Haplotype frequency | OR (95%CI) | p | |
|---|---|---|---|---|---|---|
| Case | Control | |||||
| 1 | rs2280274|rs4388726 | TA | 0.946 | 0.935 | 1.18 (0.68–2.06) | 0.559 |
| 1 | rs2280274|rs4388726 | TG | 0.978 | 0.958 | 1.64 (0.77–3.51) | 0.201 |
| 1 | rs2280274|rs4388726 | AG | 0.923 | 0.893 | 1.33 (0.85–2.09) | 0.208 |
| 2 | rs11207535|rs10889159|rs1155002 | ACT | 0.652 | 0.738 | 0.69 (0.52–0.92) | 0.011 |
| 2 | rs11207535|rs10889159|rs1155002 | GTC | 0.899 | 0.893 | 1.06 (0.69–1.60) | 0.801 |
| 2 | rs11207535|rs10889159|rs1155002 | ATC | 0.987 | 0.971 | 1.31 (0.53–3.27) | 0.561 |
| 2 | rs11207535|rs10889159|rs1155002 | ACC | 0.538 | 0.601 | 0.76 (0.58–0.99) | 0.044 |
Abbreviations: SNP, single nucleotide polymorphism; COPD, chronic obstructive pulmonary disease; OR, odds ratio; CI, confidence interval.
p<0.05 indicates statistical significance.
Bold data mean significant difference.
In this study, we investigated the associations between five SNPs in CYP2J2 and COPD risk among the Chinese Han population. Our results indicated that rs2280274, rs11207535, rs10889159 and rs1155002 were closely associated with the risk of COPD. The subgroup analysis revealed that men with GG genotype of rs11207535 and TT genotype of rs10889159 had a lower COPD risk, whereas individuals with T allele of rs1155002 significantly increased the risk of COPD. In addition, haplotype analysis showed that rs11207535, rs10889159 and rs1155002 worked together, the haplotype ACT and ACC could be the protective factors for COPD. It gives us a clue that CYP2J2 polymorphisms could be useful targets for personalized therapy on COPD.
CYP2J2 is one of CYP enzyme, which is expressed in human pulmonary endothelium.23CYP2J2 plays a vital physiological part in the pulmonary regulation of both vascular and bronchial tone.18 Recent studies indicated that CYP2J2 mediated EETs could reduce excessive inflammation in the liver and lung by using mouse models.24,25 Satoshi et al. observed that 50% reduction in CYP2J2 mRNA expression in alveolar epithelial type II cells isolated from COPD patients compared to smokers without COPD.26 Nevertheless, polymorphisms in CYP2J2 gene could affect the levels of transcription, translation, enzyme activity and result in various substances, which may affect the development of COPD. For instance, CYP2J2 rs890293 had been analyzed on the contribution to respiratory diseases among Russian and they did not find the association between CYP2J2 rs890293 and respiratory diseases.27 In this study, we found rs2280274, rs11207535 and rs10889159 decreased COPD risk, rs1155002 reversely increased the risk of COPD among the Chinese Han population, the differences with previous findings may be attributed to study population, sample size and research method. We also found that rs11207535, rs10889159 and rs1155002 were associated with COPD susceptibility in the subgroup of men. It demonstrated that CYP2J2 polymorphisms had a relationship with COPD risk and the effects of polymorphisms on COPD is gender dependent, suggesting the occurrence of COPD was related to hormone levels.
In addition, smoking has a significant association with risk of lung diseases. Therefore, we estimated the relationship of CYP2J2 polymorphisms with COPD risk. We observed a protective effect of rs2280274 on COPD risk among non-smokers. It may attribute to the lower levels inflammation from the lack of smoking. Rs11207535 increased the COPD risk of smokers, and rs1155002 had a higher risk of COPD in two groups. Our results suggest that smoking status has significant influence on COPD risk. However, our results are required to be verified by further work.
Some limitations of this study should not be ignored. First, the limitation of our sample size may influence our results, particularly in the stratified analysis. Second, several potential environmental factors were not included in this study, which may have impact on COPD risk. Third, we did not study the effects of some other diseases on COPD due to the limited information. Finally, the polymorphisms of CYP2J2 studied in this study were limited, more information on the associations between CYP2J2 gene and COPD risk needed to be studied in the future.
ConclusionOur findings suggest that polymorphisms of CYP2J2 gene are associated with COPD susceptibility and their effects on COPD risk are sex- and smoking status- specific. Further studies are required to provide accurate evidences about the influence of CYP2J2 polymorphisms on COPD risk.
Clinical implicationsThe prevalence of COPD is increasing in China. This case–control study focused on the associations of CYP2J2 polymorphisms (rs2280274, rs4388726, rs11207535, rs10889159 and rs1155002) and COPD risk. We found that rs2280274, rs11207535 and rs10889159 significantly decreased COPD risk, indicating they could protect individuals from COPD. CYP2J2 rs1155002 was associated with higher risk of COPD, suggesting it may serve as a biomarker for detecting or treating COPD. In addition, stratified analysis showed that the effects of CYP2J2 polymorphisms on COPD risk were related to gender and smoking status, it is helpful for prevention, diagnosis and individual treatment of COPD. In conclusion, our results provided information on exploring the mechanism and targeted therapy of COPD, it also promotes the development of precision medicine on COPD.
FundingThis article was financially supported by the National Natural Science Foundation of China (Nos. 81660013 and 81860015), Key Research and Development Plan of Hainan Province (No. ZDYF2018116) and Hainan Provincial Natural Science Foundation of China (No. 818QN315).
Conflict of interestsThe authors declare no conflict of interest.
We sincerely thank all participators in this study.