Currently there is lack of data regarding the impact of a home telehealth program on readmissions and mortality rate after a COPD exacerbation-related hospitalization.
ObjectiveTo demonstrate if a tele-monitoring system after a COPD exacerbation admission could have a favorable effect in 1-year readmissions and mortality in a real-world setting.
MethodsThis is an observational study where we compared an intervention group of COPD patients treated after hospitalization that conveyed a telehealth program with a followance period of 1 year with a control group of patients evaluated during one year before the intervention began. A propensity-score analyses was developed to control for confounders. The main clinical outcome was 1-year all-cause mortality or COPD-related readmission.
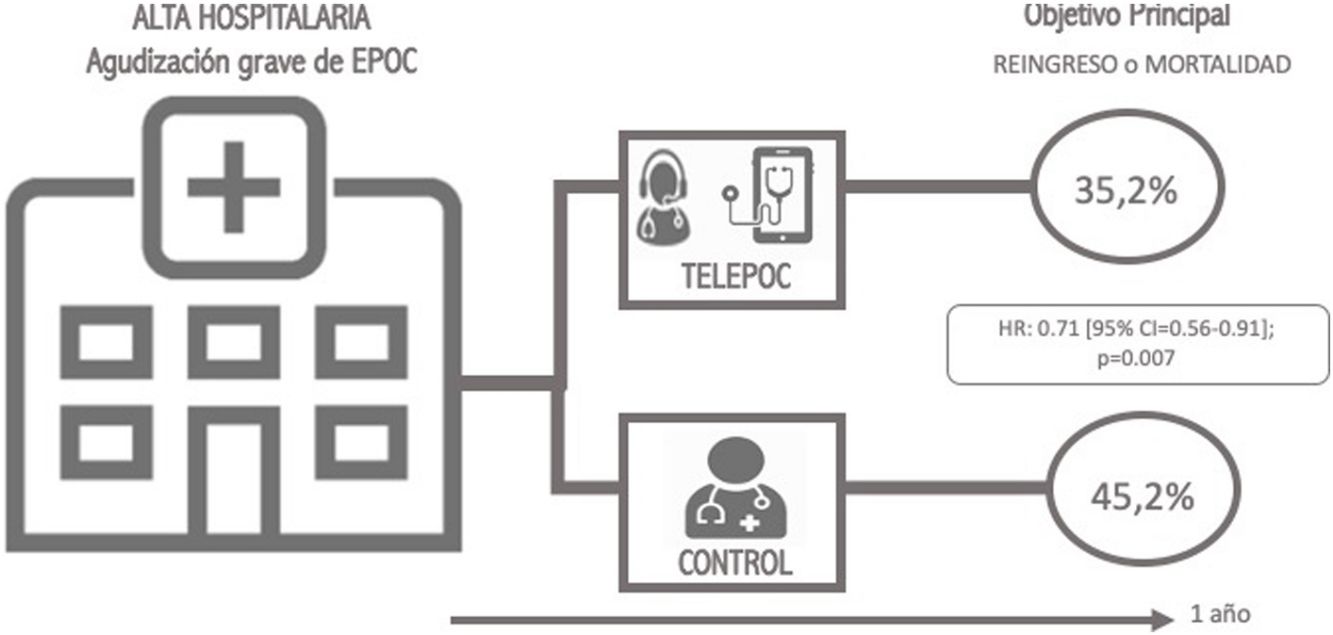
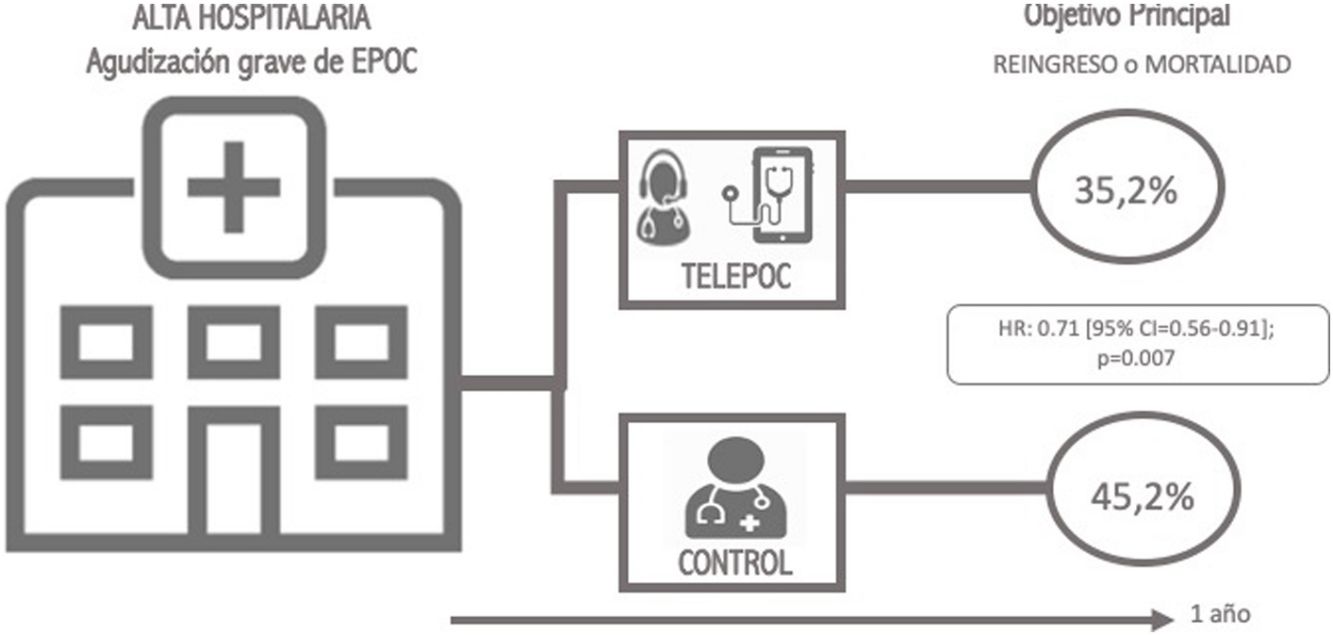
ResultsThe analysis comprised 351 telemonitoring patients and 495 patients in the control group. The intervention resulted in less mortality or readmission after 12 months (35.2% vs. 45.2%; hazard ratio [HR] 0.71 [95% CI=0.56–0.91]; p=0.007). This benefit was maintained after the propensity score analysis (HR=0.66 [95% CI=0.51–0.84]). This benefit, which was seen from the first month of the study and during its whole duration, is maintained when mortality (HR=0.54; 95% CI=[0.36–0.82]) or readmission (subdistribution hazard ratio [SHR] 0.66; 95% CI=[0.50–0.86]) are analyzed separately.
ConclusionTelemonitoring after a severe COPD exacerbation is associated with less mortality or readmissions at 12 months in a real world clinical setting.
Modern communication technologies offer new possibilities for delivering healthcare services. Telehealth for remote specialized healthcare may be used to decrease the demand on existing hospital and healthcare services,1 reducing the cost of care; measuring treatment adherence, improving accessibility and preventing re-admissions.2 The application of this modern technologies to diseases like COPD, a chronical condition which is highly prevalent3 is very attractive. It's foreseen that this pathology will become the third leading cause of death worldwide by 20204 and it is associated with significant economic burden,5,6 mostly related with the hospitalization. Consequently, an acute exacerbation actuation seems a rational approach. The importance of these programs has also been highlighted by national health systems like the Spanish in its National Strategy for COPD.7
The evidence of telehealth outcomes in the literature is contradictory.2,8 The high variability between research studies criteria and the differences in implementation of tele-monitoring services offered conflicting results from literature.9–15 So, while one of the first large randomized trials carried out in England which involved patients with COPD but also diabetes or heart failure found showed that telemedicine users had proportionally fewer hospital admissions, lower mortality, and fewer emergency admissions during the 12-month follow-up,9 another Scottish study published some months later found no significant differences in these parameters.10 Finally, a very recent meta-analysis16 showed a reduction in emergency room visits (risk ratio 0.63, 95% confidence interval 0.55–0.72) and hospitalizations (risk ratio 0.88, 95% confidence interval 0.80–0.97).
The United4Heath (U4H) Universal solutions in telemedicine deployment for European health care – project, was a pilot project under the ICT-PSP Competitiveness and Innovation Framework Program (ICT-PSP) (#325315, CIP-ICT-PSP-2012-6) supported by the European Commission. This Project targeted groups of patients with different chronic conditions: diabetes mellitus, chronic heart failure, hypertension and COPD. A large-scale pilot study was defined in order to implement a range of telehealth solutions and developed new associated care delivery processes. The COPD branch was undertaken in six trial sites across Europe (Scotland, Wales; Northern and Southern Norway; Spain and Germany). The TELEPOC study (“Teleseguimento no fogar de pacientes con Enfermidade Pulmonar Obstructiva Crónica tras a alta‿) is the Spanish arm of the study. This study was designed to validate if this telehealth care approach could be implemented at scale across different health care settings and with the hypothesis that in real world setting this kind of program could impact on morbimortality after a COPD exacerbation. Specifically, a study was carried out with the aim to demonstrate if a TM system after a COPD exacerbation admission can impact in 1-year readmissions and mortality.
MethodsStudy design and participantsThis is an observational quasi-experimental study where we compared a control group treated before the implementation of the telehealth interventions (year 2013), with an intervention group treated after the implementation of telehealth program (from February 2014 to August 2015). The intervention group was clinically tracked for 1 year. We included patients in hospital admitted due to COPD exacerbation at one of the seven secondary or tertiary hospitals from Servizo Galego de Saude (SERGAS), Galicia, Spain. This public institution covers a population of more than two and a half million people in the geographic area of Galicia. The study was approved by the regional clinical research ethics Committee (IRB# 2013/551).
Inclusion and exclusion criteriaSubjects were included in the study if (1) they were >40 years old, (2) former or active smokers (>10 pack-years), (3) had a prior spirometry with FEV1/FVC<0.7 and (4) a previous diagnosis of COPD according to the Global Obstructive Lung Diseases (GOLD) guidelines17 and a discharge diagnosis of COPD acute exacerbation according to discharge report. Patients excluded were (1) those unwilling or unable to provide written consent, (2) discharged to a locality not covered by the outreach TM team/hospital (e.g. different geographical area served by another hospital/health institution, or discharged to a new setting, i.e. from home prior to admission and discharged to a nursing home), (3) discharged to a locality with no method of electronic communication, e.g. GPRS, Wi-Fi, landline; (4) those unable or unwilling to use TM after teaching, but prior to installation and (5) at clinician's discretion due to several clinical conditions including: unreliable behavior, chaotic social circumstances, like drug or alcohol abuse, etc.
If a patient was re-admitted to the hospital more than once during the study period, only the first hospitalization was recorded and included in the analysis. After the discharge and during the follow up, patients were excluded if after repeating telephone contact, patient did not send mandatory data through TM system from a period equal or longer than 72 consecutive hours or if the patient resigned to participate into the study.
Intervention protocolTelemonitoring system and TeleCOPD groupThe aim of the COPD intervention was to support at home those patients discharged from a hospital admission due to an exacerbation, through telemonitoring and consultation in a step-down approach (high level, moderate and low). Briefly a pulmonologist team experienced in COPD and telehealth, who were trained in the local study protocol, carried out enrolment during the admission on working days in the hospital wards. Consecutive patients admitted with acute exacerbation of COPD were screened, with recruitment just prior to hospital discharge. Prior to 24h before discharge, each potential participant's medical notes were identified for inclusion and exclusion criteria by a pulmonologist. Eligible patients were duly informed about the nature and the objectives of the study. After providing written informed consent, each patient who was enrolled in the research study was supplied with a telemedicine briefcase and trained in the use of the telehealth equipment and communication system. The respiratory nurse contacted the participant by phone within 24h of discharge to confirm a signal and arrange a date/time for the first video/teleconference session.
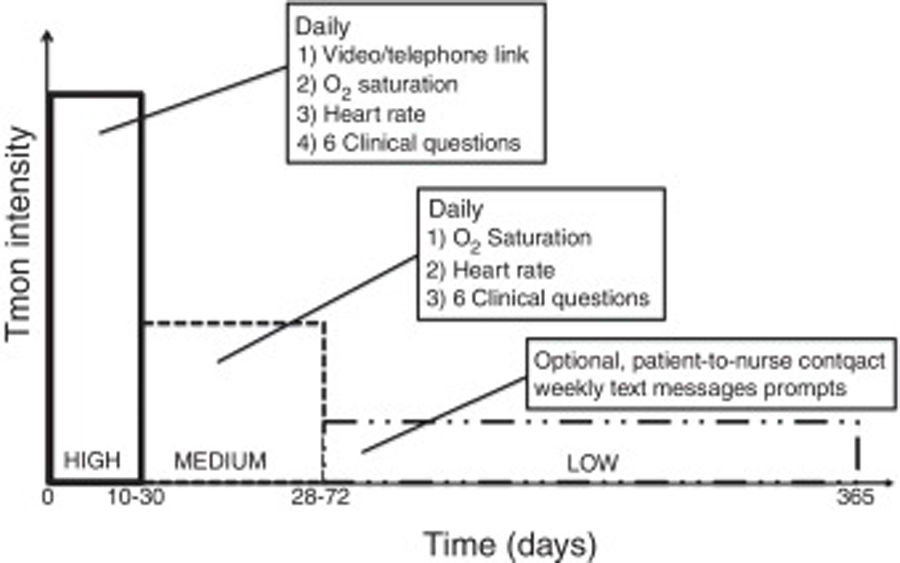
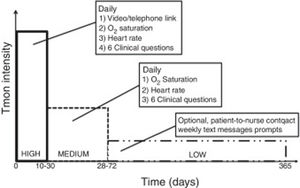
The intervention, that included clinical support services, represents three levels of intensity of telemonitoring with specific duration for each level (Fig. 1).
- 1.
High Level TM: Daily teleconsultation (preferably via video-consultation, or telephone if not possible); telemonitoring of pulse oximetry and daily symptom questions are uploaded prior to the teleconsultation, and provide a partially standardized structure to the interview. This level of TM is targeted for 10 working days (but can be a minimum of five, maximum of 30 days) after discharge to allow some pragmatism and better reflect a potential real-life clinical service.
- 2.
Moderate Level TM: Daily pulse oximetry and symptom questions uploaded for up to 12 weeks (minimum of four weeks) after discharge.
- 3.
Low Level TM: Optional patient-to-nurse contact and text message behavior prompts or website links sent to a mobile phone for up to 12 months after discharge.
During the high level of TM, a nurse made a scheduled teleconsultation (preferably video-consultation, otherwise telephone contact) with the patient after reception and review of the uploaded data through the TM system (pulse and oxygen saturation and symptom questions) from the patient that day. During the first 10 days, the clinician determined a step-down transition from the High Level to Moderate Level, or continued High Level if needed. Those needing High Level TM after 30 days, or anyone with worrisome clinical features or a combination of alerts (see below), would be referred for physician assessment, GP at primary care or specialist at hospital. After 10 working days, all patients deemed clinically stable were reviewed by the specialist nurse with the specific intention to reduce the intensity of TM to Moderate Level of TM for up to a maximum of 12 weeks, with clinical discretion to step down earlier (minimum 4 weeks) or back to Higher Level. During High and Medium level some alerts were programmed and controlled by this nurse-specialist. Alarms were set up at: heart rate below 50 bites per minute (bpm) or higher than 120bpm, oxygen saturations fall by 6% or more from their discharge baseline or when ≥two of six questions from the questionnaires were outside range (‘worse’ or ‘more than usual’) for two consecutive days. The questions and possible answers were: (1) “How do you feel today?‿:“Better‿, “As usual‿, “Worse‿; (2) “How is your breathing?‿; “Better‿, “As usual‿, “Worse‿, “Much worse‿; (3) “How is the amount of your sputum?‿: “As usual‿,‿ Worse‿, “Much worse‿; (4) “How is your sputum color?‿ “Clear/white‿, “Yellow‿, “Dark green‿ or “Brown‿; (5) “Are you using your reliever Inhalers/nebulizers or oxygen‿? “Same as usual‿, “More than usual‿, “Much more than usual‿ and (6) “Are you taking any extra antibiotics or steroids at the moment?‿ “Yes‿, “No‿. When any of this alarms were activated, the nurse-specialist took contact with the patients, evaluated their clinical situation and proceeded according to clinical protocols (continue telemonitoring, send to primary care physician, send to emergency department, send to pulmonology). During any of the telemonitoring levels, if the patient felt their symptoms get worse had the possibility to contact with the nurse-specialist through an specific telephone number during work hours, out of this hours patients had to contact with the Emergency Rescue Service (061).
Control groupIntervention patients were compared with a retrospective control group composed by a cohort of COPD patients admitted for COPD exacerbation during 2013 (the year before the TM implementation), with a similar distribution according to the number of patients included in the study of the different hospital and obstruction group based on GOLD guidelines.17
Data abstractionChart review data included demographics, comorbid conditions, basal treatment at stable state, clinical presentation at admission, laboratory data and treatment during admission. Electronic health records were employed to accurately set the date of any-cause readmission and death. Readmission and death were followed until one year after discharge.
Clinical outcomesThe main clinical outcome was a composite outcome defined as 1-year all-cause mortality or COPD-related readmission. Secondary outcomes were all-cause mortality, and COPD-related readmissions at 1-, 3-, 6-, and 12-month; time to readmission, time to death and hospital length of stay.
Completion of the study period was determined when patient completed the three levels of TM attention.
Statistical analysisCategorical variables are summarized as absolute numbers and percentages. Continuous variables are presented as means±standard deviation (SD). Normality was assessed using the Kolmogorov–Smirnov test.
For patient demographics and clinical characteristics, differences between groups were assessed using the chi-squared test or Fisher's exact test (if applicable) for categorical variables, and the unpaired Student's t-test or the Mann–Whitney U test for continuous variables.
Propensity score for each patient was calculated in a logistic regression model including all sociodemographic and clinical characteristics that resulted statistically significant in the bivariate analysis. Area under the ROC curve was computed in order to determine the discriminant ability of the propensity score to distinguish between the TeleCOPD and the control group (AUC=0.707; 95% CI=[0.67–0.74]).
In the bivariate analysis, a Kaplan–Meier approach, with the log-rank test, was employed to compare overall 12-months mortality among patients in the TeleCOPD and control group, a competing risk approach was used to estimate the cumulative incidence readmission in the 12 months following admission, treating death as a competing event, both for the TeleCOPD and for the control group. Cumulative incidences were compared using the modified log-rank test.15
To test the role of TeleCOPD in reducing mortality and readmission probability, a multivariate analysis was conducted. A Cox proportional hazards regression model was used to compare overall 12-months mortality among patients in the TeleCOPD and control group, a modified Cox regression hazard model was employed to determine the sub-distribution hazard ratios (SHRs) for readmission and their 95% confidence intervals.18 For each of these analyses, three models were run separately, comparing the (a) unadjusted regression model with both, (b) a model adjusting for variables significantly different between the study groups or significantly associated with death/readmission in the bivariate analysis, and (c) a propensity score adjusted model. Propensity score was entered as a continuous variable in the regressions.
Analysis was made by intention to treat. Data management and analyses were performed using IBM SPSS Statistics v19.0 for Windows and STATA [version 13 STATA Corporation, College Station, TX). The cumulative incidence in competing risk analyses was calculated using the cmprsk package of R.18 A two-sided p value <0.05 was considered statistically significant. The per-protocol analysis included only intervention group patients that adhered to the intervention protocol for at least 180 days.
The sample size was calculated a priori from the following assumptions: a 50% combined frequency of death or readmission at 1 year for the control group and an expected absolute reduction of this frequency from 50% to 40% with tele-monitorization therapy, in a 1:1.5 ratio. For the readmission/death estimation we employed the centralized SERGAS data regarding the admission for acute exacerbation of COPD in Galicia during 2012. A sample size of 808 patients (323 TeleCOPD and 485 control group) was necessary to detect this difference with a power of 80% and an alpha error of 0.05.
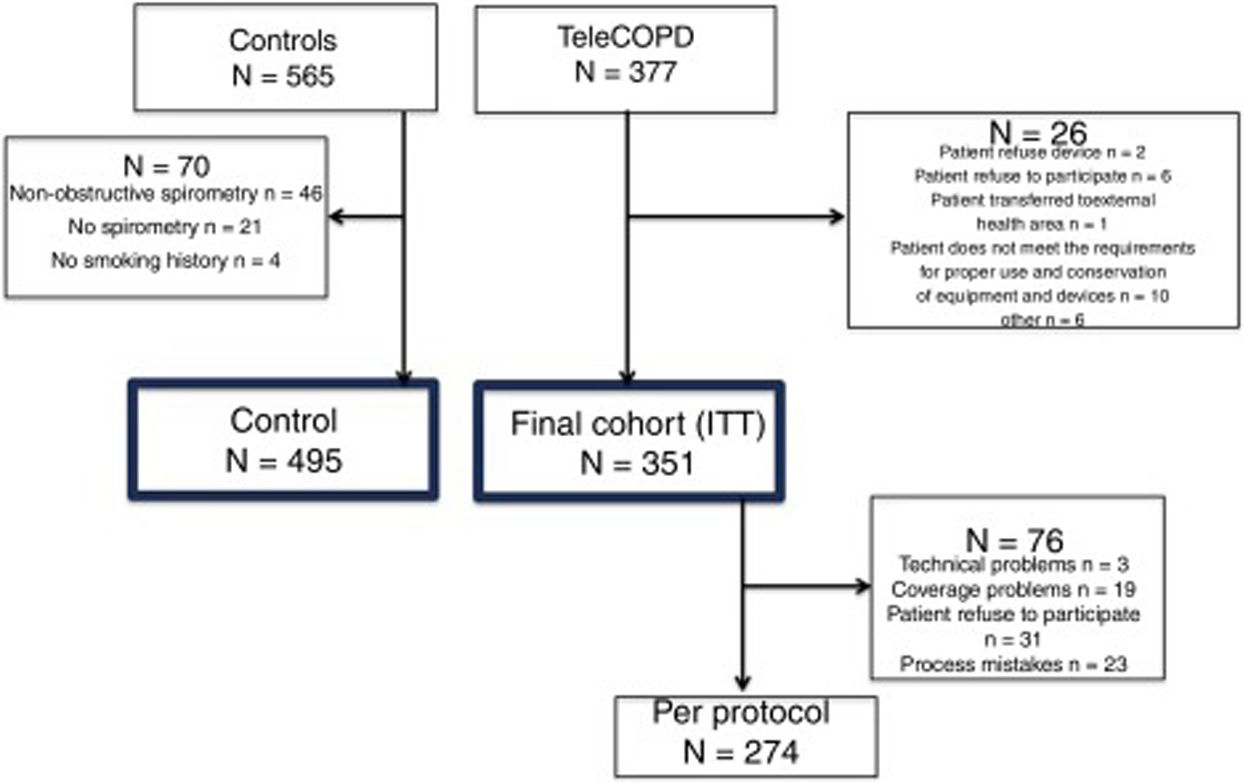
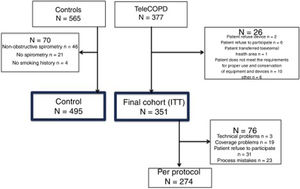
ResultsA total of 942 patients were screened, 377 from the TeleCOPD group and 565 from the control group (Fig. 2). A total of 70 control patients were excluded, mainly because the spirometry was not available or because it revealed a non-obstructive spirometry pattern, conforming a final cohort of 495 patients in the control group. Among the TeleCOPD patients, 26 where excluded for different reasons, conforming a final cohort of 351 patients who composed the TeleCOPD group. This group will be the intention to treat (ITT) group that we used for the main analysis. Among ITT group, 76 (21.6%) of the patients did not completed the study for different reasons.
Demographics, Charlson Index, respiratory function, physical and basal treatment on admission of the study population are summarized in Table 1. Compared with the control group, the TeleCOPD patients were significantly younger (68.7±9 vs. 71.3±9 years; p<0.001), had more severe COPD exacerbations during the last year (0.77±1.22 vs. 0.69±1.46; p=0.016), had more days of hospitalization during the last 12 months (6.9±11.98 vs. 6.46±15.38; p=0.015), had higher pH and heart rate at admission (7.4±0.07 vs. 7.39±0.06; p=0.01 and 82.4±13.8 vs. 79.02±14.8 p<0.001, respectively), and less cubic centimeters of FEV1 (1125.9±481.4 vs. 1208.4±514.8; p=0.013) and oxygen saturation (92.9±3.3 vs. 94±3.1%; p<0.001). The rate of invasive mechanical ventilation was higher in the TeleCOPD group (4.3% vs. 1%; p=0.002), maybe leading a higher length of hospital stay (8.85±6.01 vs. 8.21±5.93; p=0.023). Other variables where the groups were not comparable were sex and basal treatment with short acting beta agonist, long acting beta agonist, beta-blockers or phosphodiesterase-4 inhibitors (Table 1).
Demographics, COPD related history, physical and basal treatment on admission.
| Group | p-Value | ||
|---|---|---|---|
| Control(n=495) | TeleCOPD(n=351) | ||
| Age (years) | 71.3±9.4 | 68.7±9.4 | <0.001 |
| Men – n (%) | 462 (93.3) | 301 (85.8) | <0.001 |
| Smoking habit: current/former – % | 34.2/63.4 | 28.4/68.5 | 0.175 |
| Charlson index | 2.43±1.75 | 2.35±1.56 | 0.776 |
| COPD related history | |||
| Severe exacerbations | 0.69±1.46 | 0.77±1.22 | 0.016 |
| Moderate exacerbations | 1.87±2.83 | 1.64±2.2 | 0.639 |
| Total days in hospital for COPD* | 6.46±15.38 | 6.9±11.98 | 0.015 |
| FEV1% | 44.3±17.5 | 42.5±17.8 | 0.095 |
| FEV1 cc | 1208.4±514.8 | 1125.9±481.4 | 0.013 |
| GOLD CLASS*** | 0.329 | ||
| GOLD I (FEV1≥80%) | 16 (3.4) | 13 (3.7) | |
| GOLD II (50%≤FEV1<80%) | 130 (27.3) | 89 (25.6) | |
| GOLD III (30%≤FEV1<50%) | 217 (45.5) | 143 (41.2) | |
| GOLD IV (FEV1<30%) | 114 (23.9) | 102 (29.4) | |
| BMI (kg/m2) | 27.9±5.5 | 27.1±5.5 | 0.350 |
| Chronic treatment | |||
| LTOT – n (%) | 222 (44.8) | 167 (47.6) | 0.432 |
| Home NIV – n (%) | 61 (12.3) | 39 (11.1) | 0.591 |
| SABA – n (%) | 246 (49.7) | 241 (68.6) | <0.001 |
| LABA – n (%) | 426 (86.1) | 326 (92.9) | 0.002 |
| ICS – n (%) | 380 (76.8) | 293 (83.5) | 0.017 |
| Azithromycin – n (%) | 12 (2.4) | 10 (2.8) | 0.702 |
| Long-term oral steroids – n (%) | 18 (3.6) | 18 (5.1) | 0.290 |
| Statins – n (%) | 130 (26.3) | 105 (29.9) | 0.243 |
| Beta-blockers – n (%) | 25 (5.1%) | 33 (9.4) | 0.014 |
| PD-4 inhibitors – n (%) | 32 (6.5) | 37 (10.5) | 0.033 |
| Other – n (%) | 439 (88.7) | 307 (87.5) | 0.587 |
| Current admission | |||
| Respiratory failure | 436 (88.4) | 308 (87.7) | 0.760 |
| pH | 7.39±0.06 | 7.4±0.07 | 0.010 |
| SO2 | 94.0±3.1 | 92.9±3.3 | <0.001 |
| HR | 79.02±14.8 | 82.4±13.8 | <0.001 |
| Intubated – n (%) | 5 (1.0%) | 15 (4.3%) | 0.002 |
| NIV – n (%) | 107 (21.6%) | 71 (20.2%) | 0.625 |
| Length of stay (days) | 8.21±5.93 | 8.85±6.01 | 0.023 |
COPD: chronic obstructive lung disease; FEV1: exhaled volume in first second; FVC: forced vital capacity; BMI: body mass index; LTOT: long term oxygen therapy; NIV: non invasive ventilation; SABA: short acting beta-agonist; SAMA: short acting anti-muscarinic; LAMA: long acting anti-muscarinic; LABA: long acting beta agonist; ICS: inhaled corticosteroids; PD-4: phosphodiesterare; SO2: saturation of oxygen; HR: heart rate; ***GOLD: Global obstructive lung disease class.
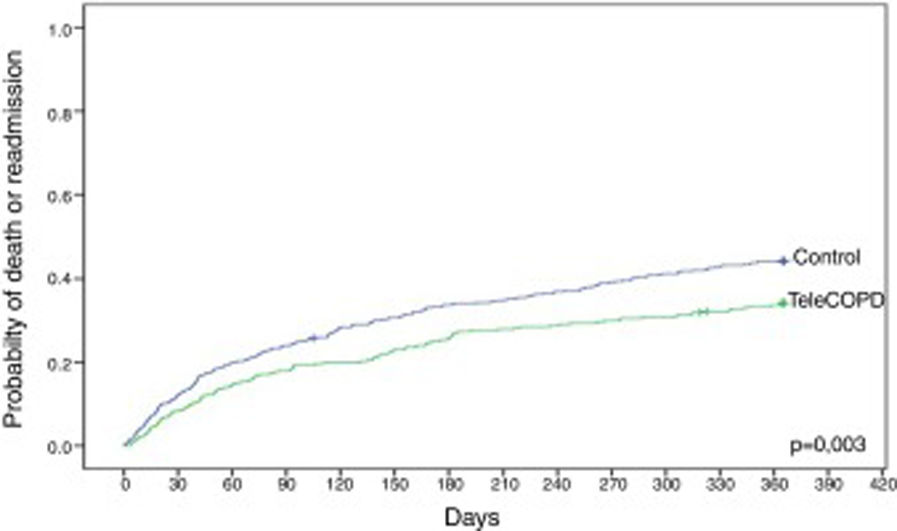
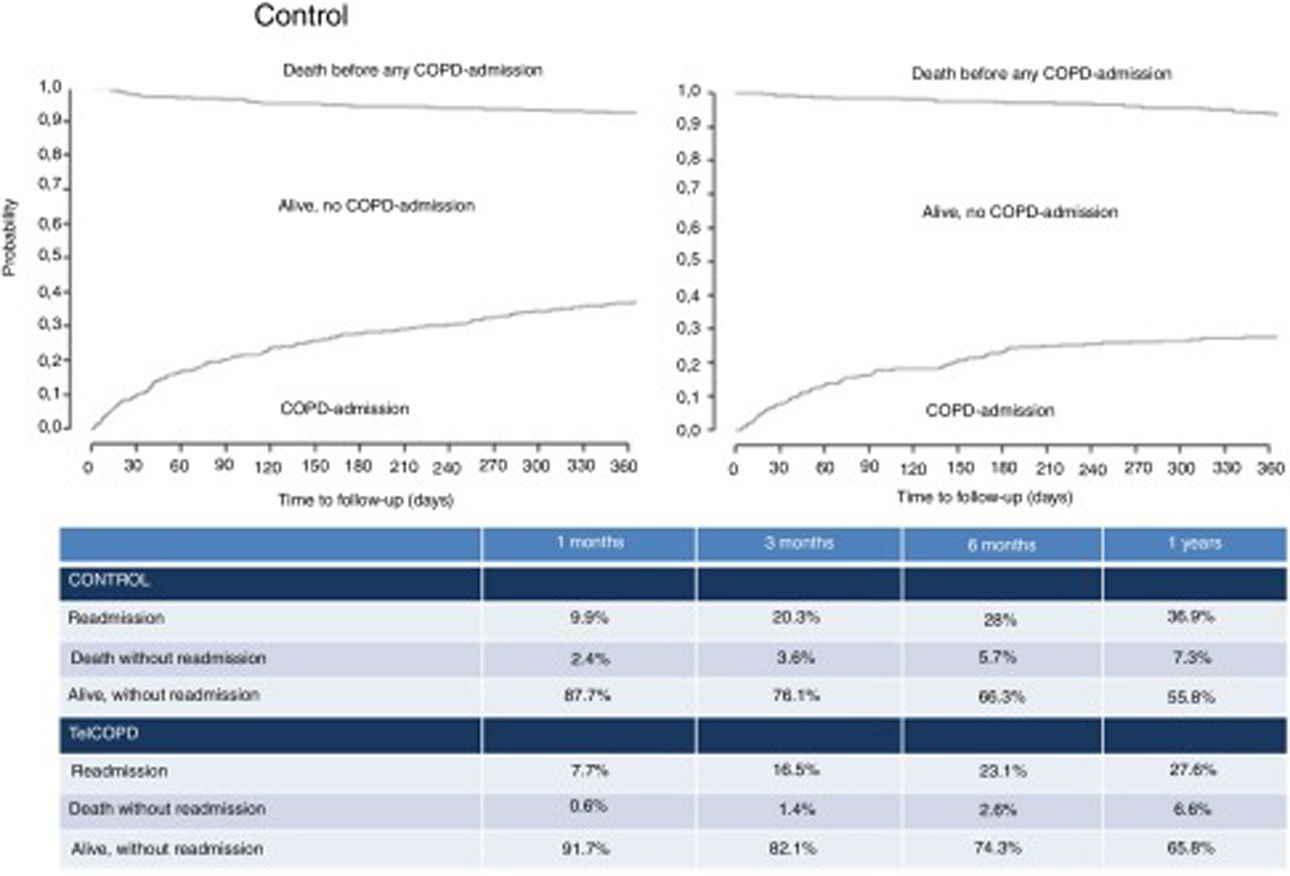
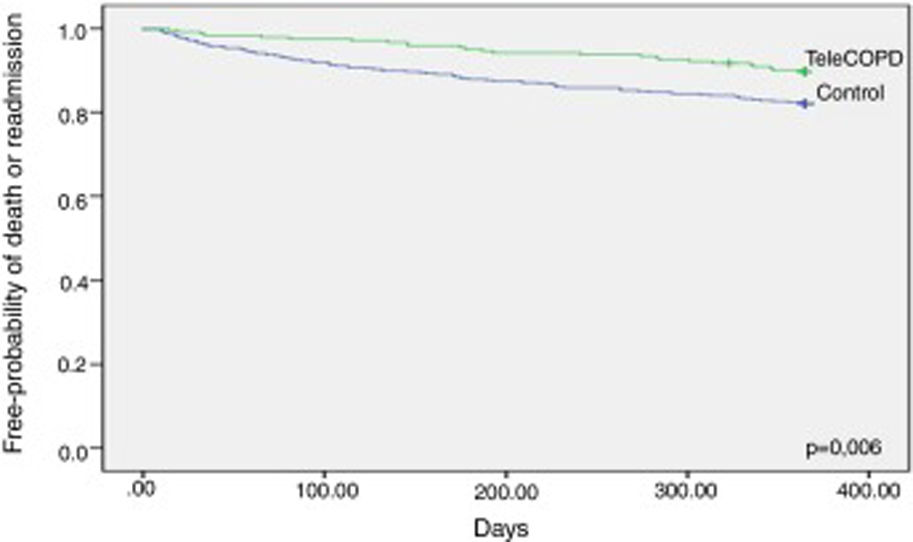
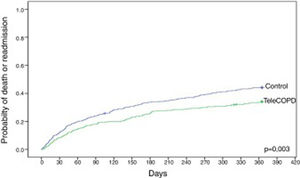
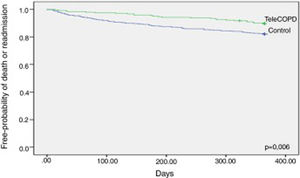
Compared with control patients, TeleCOPD patients had less significant probability of being readmitted or be dead after one year of follow up (34.2% vs. 44.3%; HR=0.71 [95% CI=0.57–0.89]; p=0.003) (Fig. 3). Accounting for death as a competing risk, the probability of readmission in the following 12 months was 27.6% for patients in the TeleCOPD group and 36.9% for patients in the control group (SHR=0.71 [95% CI=0.56–0.91]; p=0.007) (Fig. 4).
A secondary analysis was made with the aim of detecting other variables correlated with the main outcome. We compared patients who were either readmitted or who died during 12 months with those who did not. Patients readmitted or who died at 1 year were significantly older, with more comorbidities, and had higher number of exacerbations and hospitalizations, had less pulmonary function and received more frequently chronic treatment with long term oxygen, home non-invasive ventilation, inhaled antibiotics, long-term oral steroids and more cardiovascular drugs such as statins or beta-blockers (Table 2).
Sociodemographic and comorbidity variables and their association with the probability of death or readmission.
| Readmission/death | ||||
|---|---|---|---|---|
| No(n=507) | Yes(n=339) | p | Hazard ratio (95% IC) | |
| Age (years) | 69.7±9.4 | 71.0±9.6 | 0.025 | 1.01 (1.00–1.02) |
| Men – n (%) | 454 (59.5) | 309 (40.6) | 0.430 | 1.16 (0.80–1.69) |
| Charlson | 4.7±1.9 | 5.3±2.2 | <0.001 | 1.11 (1.06–1.16) |
| COPD related history | ||||
| Severe exacerbations* | 0.4±0.8 | 1.3±1.8 | <0.001 | 1.39 (1.32–1.47) |
| Moderate exacerbations* | 1.5±2.2 | 2.2±3.0 | <0.001 | 1.07 (1.04–1.11) |
| Total days in hospital for COPD* | 3.2±7.9 | 11.8±18.9 | <0.001 | 1.03 (1.02–1.03) |
| FEV1% | 45.3±18.1 | 40.9±16.6 | 0.001 | 0.99 (0.98–0.99) |
| FEV1 cc | 1243.1±530 | 1064.7±436 | <0.001 | 0.9 (0.9–1.0) |
| Chronic treatment | ||||
| LTOT – n (%) | 144 (31.6) | 195 (50.1) | <0.001 | 1.85 (1.49–2.30) |
| Home NIV – n (%) | 290 (38.9) | 49 (49) | 0.024 | 1.42 (1.05–1.92) |
| LAMA – n (%) | 49 (45.8) | 290 (39.3) | 0.159 | 0.80 (0.59–1.09) |
| LABA – n (%) | 37 (39.4) | 302 (40.2) | 0.923 | 1.02 (0.72–1.43) |
| ICS – n (%) | 63 (36.4) | 276 (41.1) | 0.303 | 1.15 (0.88–1.52) |
| NEBS – n (%) | 318 (39.2) | 21 (61.8) | <0.001 | 2.28 (1.46–3.54) |
| Azithromycin – n (%) | 330 (40.1) | 9 (40.9) | 0.936 | 0.97 (0.50–1.89) |
| Long-term oral steroids – n (%) | 315 (38.9) | 24 (66.7) | <0.001 | 2.31 (1.52–3.49) |
| Statins – n (%) | 227 (37.2) | 112 (47.7) | 0.004 | 1.40 (1.12–1.76) |
| Beta-blockers – n (%) | 305 (38.8) | 34 (58.6) | 0.005 | 1.68 (1.17–2.41) |
| PD-4 inhibitors – n (%) | 310 (39.9) | 29 (42) | 0.656 | 1.09 (0.74–1.59) |
| Other – n (%) | 26 (26) | 313 (42) | 0.004 | 1.80 (1.21–2.69) |
| Current admission | ||||
| Respiratory Failure– n (%) | 443 (59.6) | 300 (40.4) | 0.557 | 1.11 (0.79–1.55) |
| pH | 7.4±0.1 | 7.4±0.1 | 0.159 | 0.35 (0.08–1.51) |
| SO2 | 93.4±3.2 | 93.8±3.3 | 0.103 | 1.03 (0.99–1.07) |
| HR | 80.3±14.7 | 80.6±14.1 | 0.637 | 1.00 (0.99–1.01) |
| Intubated– n (%) | 15 (75) | 5 (25) | 0.219 | 0.57 (0.24–1.39) |
| NIV– n (%) | 90 (50.6) | 88 (49.4) | 0.003 | 1.44 (1.14–1.85) |
| Length of stay (days) | 8.0±5.9 | 9.1±6.0 | 0.006 | 1.02 (1.00–1.03) |
COPD: chronic obstructive lung disease; FEV1: exhaled volume in first second; FVC: forced vital capacity; BMI: body mass index; LTOT: long term oxygen therapy; NIV: non invasive ventilation; SABA: short acting beta-agonist; SAMA: short acting anti-muscarinic; LAMA: long acting anti-muscarinic; LABA: long acting beta agonist; ICS: inhaled corticosteroids; PD-4: phosphodiesterase; SO2: saturation of oxygen; HR: heart rate.
Multivariate models performed to determine the role of TeleCOPD on the risk of death and/or readmission are presented in Table 3. Independently of the outcome, and after adjusting for the propensity score, results were consistent to state that patients treated under the TeleCOPD group had between 29% and 42% less risk of readmission or death compared to those from the control group.
Unadjusted and propensity-score adjusted risk measurements for 12-months readmission and death associated to the use of TeleCOPD.
| Death | Readmission | Death or readmission | ||||
|---|---|---|---|---|---|---|
| p | HR (95% CI) | p | SHR (95% CI) | p | HR (95% CI) | |
| Model 1 | 0.006 | 0.59 (0.40–0.86) | 0.007 | 0.71 (0.56–0.91) | 0.003 | 0.71 (0.57–0.89) |
| Model 2 | 0.004 | 0.54 (0.36–0.82) | 0.003 | 0.66 (0.50–0.86) | 0.001 | 0.66 (0.51–0.84) |
| Model 3 | 0.001 | 0.47 (0.30–0.72) | 0.001 | 0.64 (0.48–0.84) | <0.001 | 0.58 (0.45–0.75) |
HR: hazard ratio; SHR: sub-distribution hazard ratio; CI: confidence interval.
-Model 1: Unadjusted regression analysis by group (TelEPOC vs. Control).
-Model 2: Regression analysis adjusted by propensity score.
-Model 3: Regression analysis adjusted by variables incorporated to the propensity score.
We also analyzed the primary outcome per protocol and results remained since the probability of being readmitted or death at 1 year was lower in the TeleCOPD group compared with the control one (10.3% vs. 18%; p=0.006) and the time to the composite outcome longer (197.8±116.3 vs. 142.7±110; HR=0.54 (95% IC=0.35–0.84) (Fig. 5).
DiscussionA specific program based on telemonitoring seems to be effective in reducing admission or death among COPD patients discharged from hospital after a severe COPD exacerbation applied in a real-world setting.
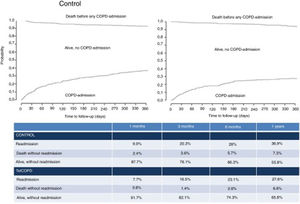
The most striking finding in this study is the positive impact that the intervention had on mortality and readmission at 12 months (35.2% vs. 45.2%; SHR=0.71 [95% IC=0.56–0.91]; p=0.007). This effect remained significant even after a propensity score analysis or even when a per-protocol analyze was done. Furthermore, when we made the distinction between readmissions and mortality we found that those differences remained (Readmission: 36.9% vs. 27.6%; Mortality: 7.3% vs. 6.6%).
Although different meta-analysis have indicated the effectiveness of telemonitoring in reducing the probability of hospitalization16,19–22 it could be considered that there are no enough data regarding the effect on mortality, as results on these studies have proved to be inconclusive or negative.19–22 However, recent studies highlights new potential benefit on survival when applying telemonitoring, especially in more severe patients.15,16 A German study which analyzed the effect of one of Europe's largest COPD telemonitoring patients found a positive impact of telemonitoring in reducing costs and healthcare utilization. Regarding mortality, it was found that a lower mortality in the intervention group (HR 0.51, 95% CI 0.30–0.86), where this reduction was even greater for the most severe cases (−3.65% [3.82 vs. 7.47%]; p<0.001). Direct comparisons between both studies must be taken cautiously, since the population was not exactly the same, patients from this study had been hospitalized during the previous two years while in our study the intervention was applied immediately after a hospitalization.
One should expect that the benefit of the intervention should be more substantial during the first days or weeks, when the intensity of the tele-monitorization was higher, oxygen and pulse were monitored and the clinical questionnaire filled. However, we observed that the benefit was maintained or even increased continuously during the 12 months. We hypothesized that this could be related to the contact with the respiratory nurse who did not only was alert from the telemonitoring data, but gave patients continuous support and education on correct disease management. The actions taken by nurses included the review of the inhalers or other treatments, leading to better adherence to their therapy and to recognize early the clinical deterioration.
So, this study also indicates that future mobile or electronic Health interventions should focus on developing self-management skills over time by providing adequate information, decision support and feedback on self-management behavior and that mHealth should complement regular care.
There are some strengths of the study that we would like to highlight. The first one is that although the TeleCOPD study was originally designed to test if it could be implemented at scale across different health care settings, we understand that a clinical approach of the data of the study could give us a strong value to test the important of telemedicine interventions in COPD patients in a real-world clinical scenario, that was the reason that led us to develop this study. So, we understand that our study reflect real-world patients that used to be slightly different to patients from randomized control trials.23 The second one is the large sample size that participated in the study, especially in the intervention arm (n=351) that is higher than other studies.24,25 Finally, the study was conducted through a long follow-up period which allowed registering and monitoring long-term clinical effects and safety data.
The reduced mortality observed in the intervention group should be confirmed with the development of a large randomized control trial with the mortality as the main outcome and it should be an important motivator to invest in these interventions and deploy to similar technologies.
However, the study has some limitations that we would like to point out. First, this study was limited to Galician population and therefore, a bias could be introduced due to specific population conditions. Since 25% of the COPD exacerbation hospital discharges are from respiratory services, it is possible that results differ when run in different patient profiles. Discharged patients from internal medicine or geriatric services where use to be older and present more comorbidities, and the TM model could not get such as positive outcomes. Third, almost a quarter of the patients selected for the TeleCOPD group were lost to follow up from the study after discharge, the most frequent reason was (1) refusing participation in the study and (2) coverage/technical problems. We believe that the reasons to give up the study after acceptation and signing the informed consent was due to patients felt overcontrolled. Another reason in older person was not having support from relatives or informal caregivers that in some cases lack technology skills. Fourth, we did not develop a cost-effective analysis, so we cannot infer that these results could impact economically. Finally, “the methodology of this non-randomized study included a historical control group, and it was based on the assumption that all the relevant co-variates had been included and that no potential confounders were missed for the analysis; however it is possible that patients who decided to participate in the intervention group might be more motivated to address their disease, or they could receive more social support that those who did not. However, we tried to minimize this kind of potential biases by constructing a propensity score analysis to verify the robustness of the results.
ConclusionIn a real-world clinical setting, telemonitoring after a severe COPD exacerbation reduces mortality and readmissions at 12 months. This benefit is maintained during the whole duration of the study. This new technology should be seen as a complement of the usual care. More studies are needed to confirm our results.
FundingICT-PSP Competitiveness and Innovation Framework Program (ICT-PSP) (#325315, CIP-ICT-PSP-2012-6) and Servicio Galego de Saude (Sergas).
Conflict of interestAll authors do not have any conflict of interest regarding the contents of this study.
PJM, CRR, CR, AFV, CZ and RG wrote and edited the manuscript. PJM, CRR, BC, SP, JQ and JC designed the study and performed statistical analysis. PJM, CRR, CR, AFV, CZ, RG, JAA, UC and AFL enrolled patients. PJM, CRR, CR, AFV, BCA, AFV, AFL, SFN, JQ, CZ, RG, JAA, UCA, SP and JGC contributed intellectually. All authors read and approved the last version of the manuscript.
We would like to thank all pulmonologist and nurses who participated in the study for their effort in developing the study over their routine clinical activities and the Sociedade Galega de Patoloxía Respiratoria, Pneumoloxía e Cirurxía Torácica (SOGAPAR) for the support. This project was cofinanced by the ICT Policy Support Program (ICT-PSP) and Servicio Galego de Saude.