CURRENT SITUATION AND LONG-TERM CONSEQUENCES OF COVID-19 INFECTION
More infoCOVID-19 is such a heterogeneous disease that a one-size-fits-all approach is not recommended, so the management of patients has been based on their clinical and laboratory characteristics.1 We therefore investigated possible homogeneous groups presenting similar features of lung involvement based on chest CT and laboratory results.
Marini and Gattinoni2 developed a model of two different phenotypes to explain lung injury in Covid-19: “Type L” and “Type H”.3 Robba et al. described three different radiological phenotypes of COVID-19 pneumonia, which is useful in preventing disease progression and improving outcome.4
On this basis we designed a study to identify a possible correlation between CT scan phenotypes, laboratory exams, and clinical outcomes. We retrospectively analysed 120 adult patients with COVID-19 5who underwent chest CT scan during hospitalisation, between March and December 2020 at our COVID-19 Hospital in two different wards: Respiratory Intensive Care Unit (RICU) and Intensive Care Unit (ICU).
The analysis of CT scans resulted in the identification of three radiological phenotypes by two blinded pulmonologists (Cohen's κ=0.9 for Phenotype 1, 0.9 for Phenotype 2 and 0.89 for Phenotype 3), in accordance with what previously described by Robba et al.4 “Phenotype 1” (PH1) is characterised by modest interstitial oedema with presentation on chest CT of diffuse ground glass opacities (GGO). “Phenotype 2” (PH2) shows predominant consolidation at lung lobes. “Phenotype 3” (PH3) shows a typical CT pattern of moderate-to-severe ARDS, with alveolar oedema.
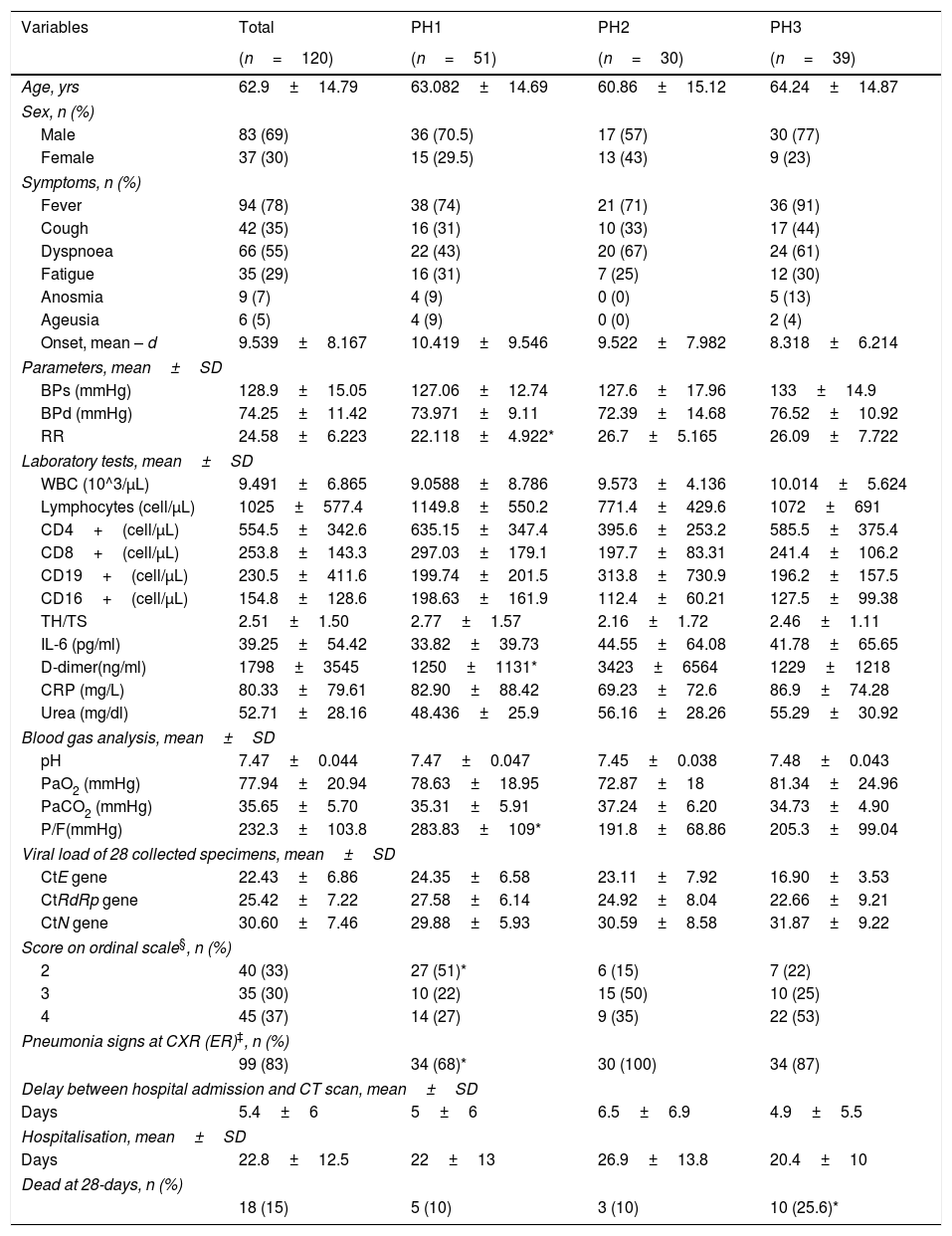
Table 1 shows demographic and clinical characteristics of the 120 patients enrolled in the study.
Demographic and Clinical Characteristics of Population.
| Variables | Total | PH1 | PH2 | PH3 |
|---|---|---|---|---|
| (n=120) | (n=51) | (n=30) | (n=39) | |
| Age, yrs | 62.9±14.79 | 63.082±14.69 | 60.86±15.12 | 64.24±14.87 |
| Sex, n (%) | ||||
| Male | 83 (69) | 36 (70.5) | 17 (57) | 30 (77) |
| Female | 37 (30) | 15 (29.5) | 13 (43) | 9 (23) |
| Symptoms, n (%) | ||||
| Fever | 94 (78) | 38 (74) | 21 (71) | 36 (91) |
| Cough | 42 (35) | 16 (31) | 10 (33) | 17 (44) |
| Dyspnoea | 66 (55) | 22 (43) | 20 (67) | 24 (61) |
| Fatigue | 35 (29) | 16 (31) | 7 (25) | 12 (30) |
| Anosmia | 9 (7) | 4 (9) | 0 (0) | 5 (13) |
| Ageusia | 6 (5) | 4 (9) | 0 (0) | 2 (4) |
| Onset, mean – d | 9.539±8.167 | 10.419±9.546 | 9.522±7.982 | 8.318±6.214 |
| Parameters, mean±SD | ||||
| BPs (mmHg) | 128.9±15.05 | 127.06±12.74 | 127.6±17.96 | 133±14.9 |
| BPd (mmHg) | 74.25±11.42 | 73.971±9.11 | 72.39±14.68 | 76.52±10.92 |
| RR | 24.58±6.223 | 22.118±4.922* | 26.7±5.165 | 26.09±7.722 |
| Laboratory tests, mean±SD | ||||
| WBC (10^3/μL) | 9.491±6.865 | 9.0588±8.786 | 9.573±4.136 | 10.014±5.624 |
| Lymphocytes (cell/μL) | 1025±577.4 | 1149.8±550.2 | 771.4±429.6 | 1072±691 |
| CD4+(cell/μL) | 554.5±342.6 | 635.15±347.4 | 395.6±253.2 | 585.5±375.4 |
| CD8+(cell/μL) | 253.8±143.3 | 297.03±179.1 | 197.7±83.31 | 241.4±106.2 |
| CD19+(cell/μL) | 230.5±411.6 | 199.74±201.5 | 313.8±730.9 | 196.2±157.5 |
| CD16+(cell/μL) | 154.8±128.6 | 198.63±161.9 | 112.4±60.21 | 127.5±99.38 |
| TH/TS | 2.51±1.50 | 2.77±1.57 | 2.16±1.72 | 2.46±1.11 |
| IL-6 (pg/ml) | 39.25±54.42 | 33.82±39.73 | 44.55±64.08 | 41.78±65.65 |
| D-dimer(ng/ml) | 1798±3545 | 1250±1131* | 3423±6564 | 1229±1218 |
| CRP (mg/L) | 80.33±79.61 | 82.90±88.42 | 69.23±72.6 | 86.9±74.28 |
| Urea (mg/dl) | 52.71±28.16 | 48.436±25.9 | 56.16±28.26 | 55.29±30.92 |
| Blood gas analysis, mean±SD | ||||
| pH | 7.47±0.044 | 7.47±0.047 | 7.45±0.038 | 7.48±0.043 |
| PaO2 (mmHg) | 77.94±20.94 | 78.63±18.95 | 72.87±18 | 81.34±24.96 |
| PaCO2 (mmHg) | 35.65±5.70 | 35.31±5.91 | 37.24±6.20 | 34.73±4.90 |
| P/F(mmHg) | 232.3±103.8 | 283.83±109* | 191.8±68.86 | 205.3±99.04 |
| Viral load of 28 collected specimens, mean±SD | ||||
| CtE gene | 22.43±6.86 | 24.35±6.58 | 23.11±7.92 | 16.90±3.53 |
| CtRdRp gene | 25.42±7.22 | 27.58±6.14 | 24.92±8.04 | 22.66±9.21 |
| CtN gene | 30.60±7.46 | 29.88±5.93 | 30.59±8.58 | 31.87±9.22 |
| Score on ordinal scale§, n (%) | ||||
| 2 | 40 (33) | 27 (51)* | 6 (15) | 7 (22) |
| 3 | 35 (30) | 10 (22) | 15 (50) | 10 (25) |
| 4 | 45 (37) | 14 (27) | 9 (35) | 22 (53) |
| Pneumonia signs at CXR (ER)‡, n (%) | ||||
| 99 (83) | 34 (68)* | 30 (100) | 34 (87) | |
| Delay between hospital admission and CT scan, mean±SD | ||||
| Days | 5.4±6 | 5±6 | 6.5±6.9 | 4.9±5.5 |
| Hospitalisation, mean±SD | ||||
| Days | 22.8±12.5 | 22±13 | 26.9±13.8 | 20.4±10 |
| Dead at 28-days, n (%) | ||||
| 18 (15) | 5 (10) | 3 (10) | 10 (25.6)* | |
Notes. All numerical data are presented as mean±SD, while categorical data as number (n) and percentage (%).
BPs: systolic blood pressure; BPd: diastolic blood pressure; CRP: c-reactive protein; IL-6: interleukin 6; PaCO2: partial pressure of carbon dioxide; P/F: ratio of the partial pressure of oxygen to the fraction of inspired oxygen; PaO2: partial pressure of oxygen; RR: respiratory rate; WBC: white blood cell.
Seven-category ordinal severity scale: no hospitalisation or discharged=1; hospitalisation, not requiring supplemental oxygen=2; hospitalisation, requiring supplemental oxygen by mask or nasal cannula=3; hospitalisation, requiring high-flow oxygen (HFO) or non-invasive ventilation (NIV)=4; hospitalisation, requiring invasive mechanical ventilation=5; hospitalisation, ventilation and additional organ support – pressors, RRT, ECMO=6; death=7.
Chest CT scans were acquired on average at 5 (±6 SD) days after hospital admission. Based on the results of CT scans, 51 out of 120 were classified as phenotype 1, 30 as phenotype 2 and 39 as phenotype 3.
The analysis of laboratory exams at admission, 14 and 28 days showed no difference among the groups, except D-dimers that showed a statistically significant higher level for PH2 (3423±6564ng/ml, at admission; 3058.6±5466 at 14 days and 1037.9±794at 28 days) than in PH1 and PH3 at all time points, while there was not statistical difference between PH3 and any other phenotype.
This could be explained by the different pathophysiological mechanisms underlying each phenotype, specifically according to a different prevalence of coagulopathy. The high prevalence of coagulopathy and venous thromboembolism in Covid-19 may contribute to respiratory deterioration. In critically ill COVID-19 patients, a local direct vascular and endothelial injury produces microvascular clot formation and angiopathy In the microcirculation of the lung and potentially other organs. In the initial phase of the infection, D-dimer and fibrinogen levels are increased, while activated partial prothrombin time, prothrombin time, and platelet counts are often relatively normal. Increased D-dimer levels three times the upper limit of normal may trigger screening for venous thromboembolism.6 Therefore, according to our results, it is possible that patients with phenotype 2 (showing a higher level of D-dimer) have a higher burden of vascular damage than the others where alveolar injury seems to be more prevalent as shown by the prevalence of ground glass.
Blood gas analysis showed statistically different PaO2/FiO2 ratio between PH1 with both other phenotypes (P<.01 each), at baseline, while no difference between PH2 and PH3 (P=.08) was observed.
At hospital admission the severity of disease was measured by seven-category ordinal scale and showed significant differences between the various phenotypes; in particular, PH1 showed the highest percentage (52.5%) of subjects who did not require supplemental oxygen on hospital admission. Ordinal Score was monitored during hospitalisation: in PH3 the majority of patients underwent non-invasive ventilation (72%, 28/39, vs 60%, 18/30 of PH2, and 51%, 26/51 of PH1). In PH2 and PH3 groups, all patients required at least O2 supplementation, while most of PH1 patients did not need O2 supplementation or ventilation. Considering the need for oxygen therapy throughout the hospitalisation, 11 patients in the PH1 group (21.5%) never needed oxygen therapy (40 patients did, instead), while all PH2 and PH3 patients were treated with oxygen at a certain point. More precisely, in PH1 group, 14/40 patients were treated with low flow oxygen therapy, while 26/40 (65% of oxygen-treated) needed HFO/NIV/IMV. In PH2, 12/30 patients were treated with low flow oxygen, while 18/30 (60%) needed HFO/NIV/IMV. In PH3, 11/39 patients needed low flow oxygen, while 28/30 (72%) needed HFO/NIV/IMV. This difference in ventilatory support needed by the various phenotypes could be explained by the different pathophysiological mechanism underlying the three patterns, with PH1 and PH3 apparently related to alveolar oedema and PH2 to the dead-space effect.
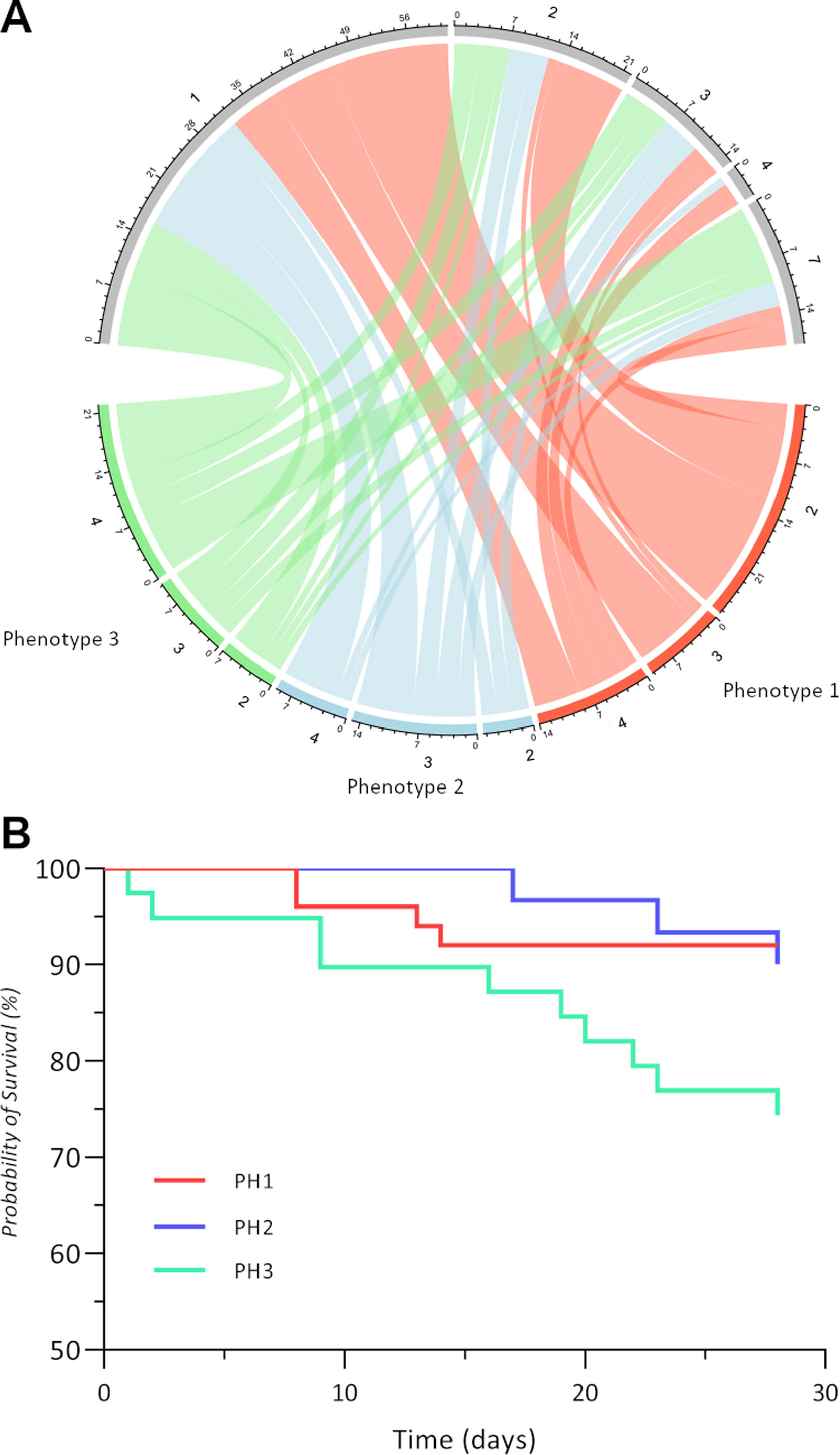
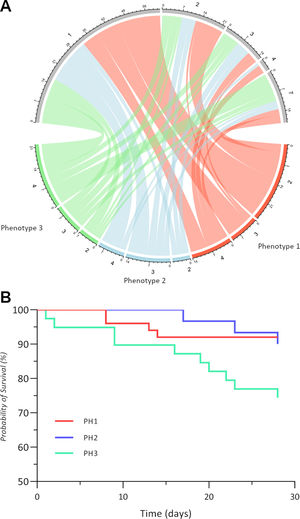
The outcome of all phenotypes was evaluated at 28-day, as shown in Fig. 1A.
Outcomes of three radiological phenotypes at 28 days. (A) Chord diagram illustrates the ordinal severity scale of the three phenotypes: phenotype 1 is red, phenotype 2 is blue, and phenotype 3 is green. For each phenotype, the severity scale was assessed at admission and after 28 days and is connected by a ribbon whose width is dependent on the number of patients who are different from those in the derivation cohort. (B) Kaplan–Meier curves of each phenotype survival at 28 days after hospital admission.
The largest number of discharged patients was from PH1 (29/51) in contrast to the PH2 (16/30) and PH3 (16/39) groups. At 28 days, 7 hospitalised patients of PH3 did not require supplemental oxygen versus 10 PH1 and 5 PH2, while none of PH3 required HFO or NIV in contrast to 3 of PH1 and 1 of PH2.
Most patients who showed PH1 (24%, 29/120 patients) were discharged from hospital and did not require supplemental oxygen (22.5%, 27/120 patients) on admission to hospital. Therefore, this phenotype might be associated with better outcomes. In PH1 lung compliance is preserved or even elevated and the amount of the gas in the lung is nearly normal, while the amount of non-aerated tissue is very low.7 Gattinoni et al. hypothesised that the primary cause of hypoxaemia in type-L is perfusion defects presumably caused by vasoconstriction and high shunt fraction. In contrast, the high elastance in type-H is thought to be induced by lung oedema.3
Deaths were 25.6% (10/39) in PH3 versus 10% (5/51) in PH1 and 10% (3/30) in PH2 at 28 days.
The mortality rate of the population is estimated at 15% with a significantly worse trend in the PH3 group (P=.04) (Fig. 1B).
At 28 days, 18 patients had died. Phenotype 3 had higher mortality (8% overall, 10/120) than the group showing only GGOs (4%, 5/120) or only consolidations (2.5%, 3/120) at CT scan. This phenotype is characterised by an increased oedema with a decreased gas volume and an increased amount of non-aerated tissue and for this reason they require more ventilatory support.7 As Most patients who died required already HFO or NIV (55.5%, 10/18) at admission, we cannot exclude a possible link between these deaths and ventilator-induced lung injury (VILI)8 as in the “baby lung” model.9
Based on our results, we could hypothesise that phenotype 2 shows a different trend from all the others and would seem to be more related to a coagulopathy, although we cannot exclude the hypothesis that one phenotype evolves from the other. Further studies might focus on the predictive role of D-dimer, and its cut-offs, in delineating the PH2 patients, that could require an early CT scan to avoid excessive pressure support and finally prevent VILI. To further understand the exact basis of the different CT scan phenotype, a longer longitudinal analysis of clinical and laboratory features (e.g., timing of weaning, pressures and FiO2 delivered) in each phenotype and a comparison among them is needed.
FundingThe authors did not receive any funds to disclose.
Conflict of InterestsThe authors have no conflict of interests to declare.
The authors would like to thank Dr. E.V.M. Sassani for his work in reporting the radiological investigations of our practice. Thanks also to all the health professionals at the U.H. Policlinico Riuniti in Foggia who have been involved in the care and assistance of COVID-19 patients for over a year.