CURRENT SITUATION AND LONG-TERM CONSEQUENCES OF COVID-19 INFECTION
More infoThe activity of lung transplant programmes has been significantly affected since the beginning of the COVID-19 pandemic by a drop in the number of donors and the surgical capacity of hospital centres. During the lockdown period, organ donation was almost paralysed due to the saturation of intensive care units and the use of operating theatres as care units for critically ill patients. The cyclic evolution of the pandemic and the rapid response from the National Transplant Organization managed to sustain lung transplant activity. Currently, many programs have almost fully recovered the pre-pandemic activity (Fig. 1). However, the pandemic is still having an especially negative impact on lung transplant recipients. The airborne transmission of the virus coupled with the characteristics of lung grafts and immunosuppression make lung transplant recipients especially vulnerable to both infection and the development of severe forms of the disease. The mortality associated with pneumonia due to severe acute respiratory SARS-CoV-2 syndrome in these patients can be in excess of 40%1–3 and is a clear reflection of the ineffectiveness of the prevention and treatment measures applied to now.
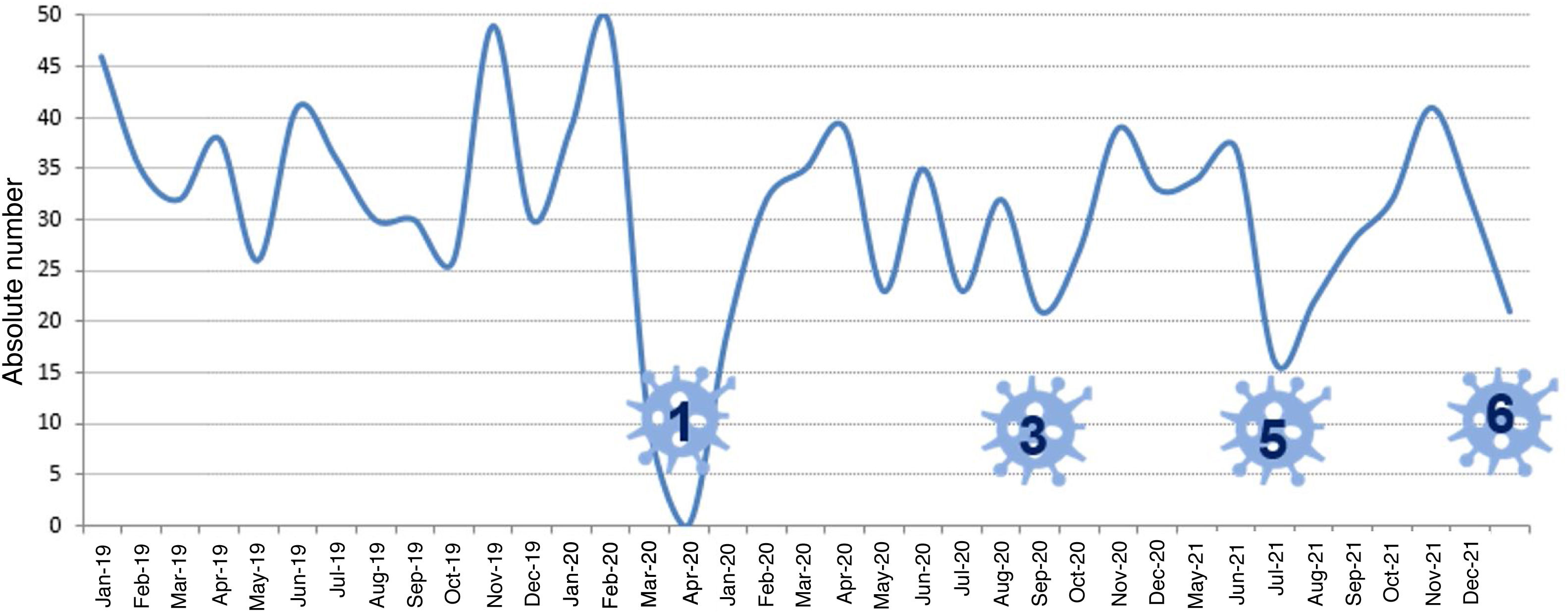
Lung transplant activity in Spain during the COVID-19 pandemic. Evolution of transplantation activity in Spain between 2019 and 2021. During the lockdown period, organ donation was almost paralysed due to the saturation of intensive care units. The impact of the following waves on transplantation activity was lower due to the response of the National Transplant Organisation and the geographical distribution of infection (graph provided by the National Transplant Organisation). The greatest impact on transplant activity occurred in waves 1, 3, and 5, represented by numbers in the figure.
Vaccination is necessary, but is not enough to protect these patients.4 Fewer than half of transplant recipients generate a protective response despite repeated vaccination cycles.5 The factors associated to this reduced vaccine response are the intensity of immunosuppression, hypogammaglobulinaemia, and the use of anti-metabolites — especially mycophenolate. It is therefore advisable for lung transplant recipients to maintain the adherence to basic personal protection measures such as FFP2 face masks, hand hygiene, social distancing and the adequate ventilation of closed spaces.
The protection of patients with little or no vaccine response may significantly improve with the availability of long-acting monoclonal antibodies. Evusheld® is a combination of two long-acting antibodies (tixageviman-cilgavimab) recently approved by the Food and Drug Administration for pre-exposure prophylaxis of patients without vaccine response.6 The intramuscular availability of the drug facilitates the administration at the outpatient level. Evusheld® confers six-month protection against infection from different strains of SARS-CoV-2, including Delta. The level of protection against Omicron is unknown because the clinical trial was performed when this variant was not yet prevalent. The drug should be administered every 6 months in case of pandemic persistence.
For patients in the initial stages of the disease, the only therapeutic tools available have been the temporarily reduction of immunosuppression, the dose adjustments of corticosteroids and/or prophylactic anticoagulation. Progressive forms of the disease have been treated with remdesivir, bolus administration of steroids, immunomodulators and advanced respiratory support with uncertain results. Fortunately, the therapeutic armamentarium to prevent disease progression has been significantly increased in recent months with the incorporation of effective antivirals and monoclonal antibodies. The risk of hospitalisation and death is significantly reduced with the early administration of remdesivir, the nirmatrelvir-ritonavir combination (Paxlovid®) or molnupiravir.7–9
Monoclonal antibodies are also effective to prevent progression, but the efficacy of many has been reduced with the emergence of the Omicron variant. Sorovimab was the only antibody that maintains activity against all strains of SARS CoV-2 currently circulating at the time of writing this article, due to its design based on an ancestral epitope of the pan-sarbecovirus family.10
We have no data from clinical trials on immunosuppressed patients, but favourable clinical experiences have been reported with the early administration of monoclonal antibodies in liver and kidney transplant recipients.11 In a hypothetical scenario with unlimited availability of drugs, the choice of the treatment should be personalised according to the patient's characteristics. However, some drugs are not yet available or are scarce. Other limitations to take into account are in relation to the logistics required for the administration of the drugs and their potential side effects or pharmacological interactions. The need for venous access may condition the use of monoclonal antibodies and remdesivir. The availability of effective oral antivirals such as Paxlovid® and molnupiravir will facilitate administration in the initial stages of the disease. However, the ritonavir content of Paxlovid® can induce a lot of hard-to-manage drug interactions in transplant recipients. Molnupiravir offers less protection against progression of the disease, and the mechanism of action of the drug at the viral genome level contraindicates its administration in adolescents and pregnant women.
Transplant units should facilitate patient access to these effective drugs in the early stages of the disease. However, follow-up of our recipients has been significantly disrupted by the collapse of departments of pneumology and the patients’ fear of contracting the disease. Transplant units have had to adapt the clinical activity to the requirements imposed by the pandemic. Follow-up telephone visits, home control of pulmonary function and consultation emails have become common practices. Some of these tools are here to stay, so it is essential to assess them properly. From an efficiency point of view, the pandemic could be considered an opportunity to evaluate, learn and improve our activity.
The incorporation of new technologies of information and communications (ICTs) in post-transplant follow-up may improve the accessibility of and for patients. ICTs could also strengthen the interactions between professionals from different centres involved in their care. These technologies have been shown to be feasible and effective for the remote control of pulmonary function and the monitoring of adherence to immunosuppressive treatment in lung transplant recipients.12,13 Transplant units with active tele-health applications have managed to maintain routine follow-up of their patients during the pandemic period in close collaboration with community health care centers.14 The education of patients and professionals could also improve from training delivered through ICTs. E-learning is a training tool with flexibility of use in time, number and space that can be very useful to improve the empowerment and self-management of lung transplant recipients.15
The last 2 years of COVID-19 pandemic have taken the lives of too many patients. Moreover, the weaknesses of our health care system and the chronic shortage of resources have been made evident. There are optimistic predictions foreseeing the end of the pandemic, but the Omicron variant has proven that we are still at risk. Therefore, we must remain alert and prepared to deliver the best available care during the pandemic waves as well as the normal daily activity. Current medical care is based on direct interactions between patients and physicians. The development of hybrid care systems could combine the advantages of personal contact with the efficiency of ICTs.
Health managers, professionals and patients should all get involved in the implementation of new hybrid care systems whilst preserving the warmth of personal interaction. These systems should facilitate the access of the patients to the best care available, while guaranteeing quality, equity and efficiency.