Patients with bronchiectasis present exacerbations which often require hospital admissions. These exacerbations are associated with elevated morbidity and increased medical costs.1 Several scores to predict mortality bronchiectasis outpatient population have been validated2,3 and the influence of comorbidities on mortality and outcomes of patients with bronchiectasis have been assessed.4,5 Our aim was to determine predictors of poor outcomes in patients admitted for exacerbation of bronchiectasis, focusing on the impact of comorbidities on hospital stay, in-hospital mortality and readmissions.
This was an observational prospective study conducted in the Germans Trias i Pujol University Hospital, in Badalona (Barcelona, Spain). All admissions with a diagnosis according to the International Classification of Diseases “Bronchiectasis with acute exacerbation”, between 2013 and 2021 were initially included in the analysis. The endpoints were days of hospitalization, in-hospital mortality, and hospital readmissions within 30 days after discharge. Bronchiectasis Severity Index (BSI), E-FACED, Bronchiectasis Aetiology Comorbidity Index (BACI) and Charlson comorbidity index were calculated for each one. The association between clinical variables and categorical outcomes was assessed using logistic regression. The association between clinical variables and quantitative outcomes was assessed with linear regression. To define a long hospital stay we arbitrarily used 75th percentile. To analyze the predictive value of scores such as BSI, E-FACED, BACI or Charlson, ROC area was used. The equality of ROC areas was tested using the rocgold command of STATA. For each outcome, we used the scale with a large ROC area as the gold standard. For each comparison, rocgold reported the raw and the Bonferroni-adjusted significance probability.
The research protocol was approved by the regional ethics committee (Ethics Committee for Clinical Research of the XXX, with reference PI-13-053.
Finally, 143 patients with 248 admissions due to exacerbation of bronchiectasis were included in our study. All the patients were followed up 30 days after discharge to confirm the presence of readmissions.
The mean age for the whole cohort was 73.4 years; 99 (69.2%) were female with a Pseudomonas aeruginosa (PA) chronic infection rate of 28% and a median predicted FEV1 of 60.7%. Mean BSI, E-FACED, BACI and Charlson comorbidity index were, respectively, 7.7, 2.3, 6.2 and 2.7. Twenty six different comorbidities were reported in this cohort with a heavy-tailed distribution, ranging from a prevalence of 88% for arterial hypertension to a 4% for inflammatory bowel disease.
Mean hospital stay was 8.2 days and we defined long hospital stays of 11 days or longer. Patients with a higher degree of dyspnea and those with a greater E-FACED score had longer hospitalizations. Among comorbidities chronic heart failure, diabetes mellitus, osteoarthrosis and previous stroke were associated with an increased hospital stay (Table 2). Twenty-nine (11.7%) hospitalizations were followed by a readmission within 30 days. Patients more functionally independent as measured by Barthel index were less likely to be readmitted. No other factor or comorbidity was associated with the risk of readmission. Fourteen patients (5.7%) died during the hospitalization and the risk factors are described in Table 1. Hypercapnia on admission was the only statistically significant risk factor identified for in-hospital mortality. E-FACED score, BACI and Charlson Index had a similar predictive power for longer stay in this cohort with an AUC of 0.64 (95% CI 0.55–0.73), 0.63 (95% CI 0.54–0.72) and 0.61 (95% CI 0.52–0.70), respectively, versus 0.54 (95% CI 0.45–0.63) for BSI. For readmission in 30 days, all the prognostic scores had the similar predictive value with an AUC of 0.64 (95% CI 0.52–0.77) for E-FACED score, 0.61 (95% CI 0.51–0.72) for BACI, 0.60 (95% CI 0.47–0.73) for BSI and 0.59 (95% CI 0.47–0.72) for Charlson Index. The BSI had the highest mortality predictive ability in this cohort with an AUC of 0.78 (95% CI 0.65–0.91) versus 0.58 (95% CI 0.36–0.80) for the E-FACED score, 0.58 (95% CI 0.28–0.87) for BACI and 0.41 (95% CI 0.06–0.78) for Charlson Index.
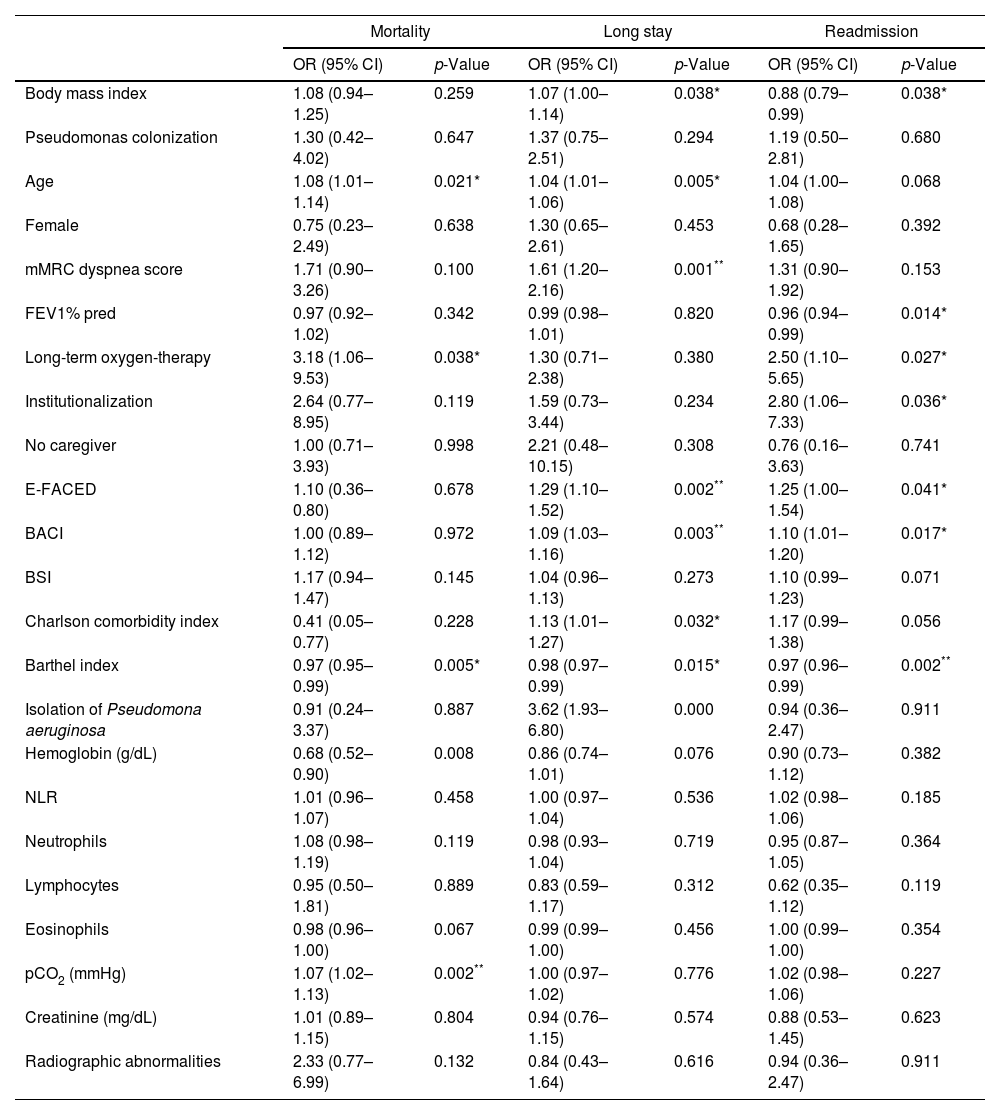
Patient's characteristics and outcomes.
| Mortality | Long stay | Readmission | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Body mass index | 1.08 (0.94–1.25) | 0.259 | 1.07 (1.00–1.14) | 0.038* | 0.88 (0.79–0.99) | 0.038* |
| Pseudomonas colonization | 1.30 (0.42–4.02) | 0.647 | 1.37 (0.75–2.51) | 0.294 | 1.19 (0.50–2.81) | 0.680 |
| Age | 1.08 (1.01–1.14) | 0.021* | 1.04 (1.01–1.06) | 0.005* | 1.04 (1.00–1.08) | 0.068 |
| Female | 0.75 (0.23–2.49) | 0.638 | 1.30 (0.65–2.61) | 0.453 | 0.68 (0.28–1.65) | 0.392 |
| mMRC dyspnea score | 1.71 (0.90–3.26) | 0.100 | 1.61 (1.20–2.16) | 0.001** | 1.31 (0.90–1.92) | 0.153 |
| FEV1% pred | 0.97 (0.92–1.02) | 0.342 | 0.99 (0.98–1.01) | 0.820 | 0.96 (0.94–0.99) | 0.014* |
| Long-term oxygen-therapy | 3.18 (1.06–9.53) | 0.038* | 1.30 (0.71–2.38) | 0.380 | 2.50 (1.10–5.65) | 0.027* |
| Institutionalization | 2.64 (0.77–8.95) | 0.119 | 1.59 (0.73–3.44) | 0.234 | 2.80 (1.06–7.33) | 0.036* |
| No caregiver | 1.00 (0.71–3.93) | 0.998 | 2.21 (0.48–10.15) | 0.308 | 0.76 (0.16–3.63) | 0.741 |
| E-FACED | 1.10 (0.36–0.80) | 0.678 | 1.29 (1.10–1.52) | 0.002** | 1.25 (1.00–1.54) | 0.041* |
| BACI | 1.00 (0.89–1.12) | 0.972 | 1.09 (1.03–1.16) | 0.003** | 1.10 (1.01–1.20) | 0.017* |
| BSI | 1.17 (0.94–1.47) | 0.145 | 1.04 (0.96–1.13) | 0.273 | 1.10 (0.99–1.23) | 0.071 |
| Charlson comorbidity index | 0.41 (0.05–0.77) | 0.228 | 1.13 (1.01–1.27) | 0.032* | 1.17 (0.99–1.38) | 0.056 |
| Barthel index | 0.97 (0.95–0.99) | 0.005* | 0.98 (0.97–0.99) | 0.015* | 0.97 (0.96–0.99) | 0.002** |
| Isolation of Pseudomona aeruginosa | 0.91 (0.24–3.37) | 0.887 | 3.62 (1.93–6.80) | 0.000 | 0.94 (0.36–2.47) | 0.911 |
| Hemoglobin (g/dL) | 0.68 (0.52–0.90) | 0.008 | 0.86 (0.74–1.01) | 0.076 | 0.90 (0.73–1.12) | 0.382 |
| NLR | 1.01 (0.96–1.07) | 0.458 | 1.00 (0.97–1.04) | 0.536 | 1.02 (0.98–1.06) | 0.185 |
| Neutrophils | 1.08 (0.98–1.19) | 0.119 | 0.98 (0.93–1.04) | 0.719 | 0.95 (0.87–1.05) | 0.364 |
| Lymphocytes | 0.95 (0.50–1.81) | 0.889 | 0.83 (0.59–1.17) | 0.312 | 0.62 (0.35–1.12) | 0.119 |
| Eosinophils | 0.98 (0.96–1.00) | 0.067 | 0.99 (0.99–1.00) | 0.456 | 1.00 (0.99–1.00) | 0.354 |
| pCO2 (mmHg) | 1.07 (1.02–1.13) | 0.002** | 1.00 (0.97–1.02) | 0.776 | 1.02 (0.98–1.06) | 0.227 |
| Creatinine (mg/dL) | 1.01 (0.89–1.15) | 0.804 | 0.94 (0.76–1.15) | 0.574 | 0.88 (0.53–1.45) | 0.623 |
| Radiographic abnormalities | 2.33 (0.77–6.99) | 0.132 | 0.84 (0.43–1.64) | 0.616 | 0.94 (0.36–2.47) | 0.911 |
mMRC: modificate Medical Research Council; FEV1: forced expiratory volume in 1s; % of predicted value; BACI: Bronchiectasis Aetiology and Comorbidities Index; BSI: bronchiectasias severity index; NLR: neutrophil-to-lymphocite ratio; pCO2: carbon dioxide partial pressure; OR: odds ratio; CI: confidence interval.
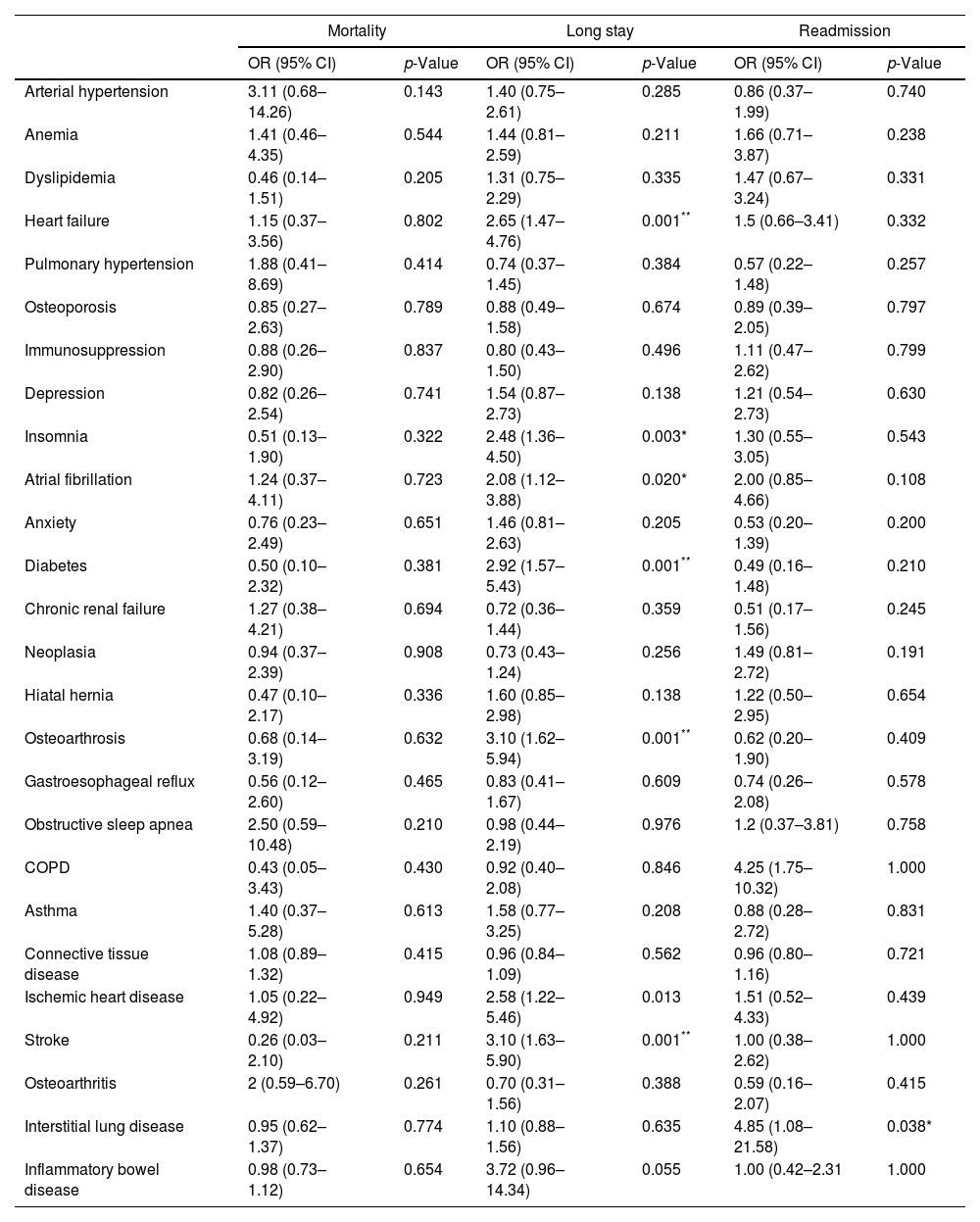
Comorbidities and outcomes.
| Mortality | Long stay | Readmission | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Arterial hypertension | 3.11 (0.68–14.26) | 0.143 | 1.40 (0.75–2.61) | 0.285 | 0.86 (0.37–1.99) | 0.740 |
| Anemia | 1.41 (0.46–4.35) | 0.544 | 1.44 (0.81–2.59) | 0.211 | 1.66 (0.71–3.87) | 0.238 |
| Dyslipidemia | 0.46 (0.14–1.51) | 0.205 | 1.31 (0.75–2.29) | 0.335 | 1.47 (0.67–3.24) | 0.331 |
| Heart failure | 1.15 (0.37–3.56) | 0.802 | 2.65 (1.47–4.76) | 0.001** | 1.5 (0.66–3.41) | 0.332 |
| Pulmonary hypertension | 1.88 (0.41–8.69) | 0.414 | 0.74 (0.37–1.45) | 0.384 | 0.57 (0.22–1.48) | 0.257 |
| Osteoporosis | 0.85 (0.27–2.63) | 0.789 | 0.88 (0.49–1.58) | 0.674 | 0.89 (0.39–2.05) | 0.797 |
| Immunosuppression | 0.88 (0.26–2.90) | 0.837 | 0.80 (0.43–1.50) | 0.496 | 1.11 (0.47–2.62) | 0.799 |
| Depression | 0.82 (0.26–2.54) | 0.741 | 1.54 (0.87–2.73) | 0.138 | 1.21 (0.54–2.73) | 0.630 |
| Insomnia | 0.51 (0.13–1.90) | 0.322 | 2.48 (1.36–4.50) | 0.003* | 1.30 (0.55–3.05) | 0.543 |
| Atrial fibrillation | 1.24 (0.37–4.11) | 0.723 | 2.08 (1.12–3.88) | 0.020* | 2.00 (0.85–4.66) | 0.108 |
| Anxiety | 0.76 (0.23–2.49) | 0.651 | 1.46 (0.81–2.63) | 0.205 | 0.53 (0.20–1.39) | 0.200 |
| Diabetes | 0.50 (0.10–2.32) | 0.381 | 2.92 (1.57–5.43) | 0.001** | 0.49 (0.16–1.48) | 0.210 |
| Chronic renal failure | 1.27 (0.38–4.21) | 0.694 | 0.72 (0.36–1.44) | 0.359 | 0.51 (0.17–1.56) | 0.245 |
| Neoplasia | 0.94 (0.37–2.39) | 0.908 | 0.73 (0.43–1.24) | 0.256 | 1.49 (0.81–2.72) | 0.191 |
| Hiatal hernia | 0.47 (0.10–2.17) | 0.336 | 1.60 (0.85–2.98) | 0.138 | 1.22 (0.50–2.95) | 0.654 |
| Osteoarthrosis | 0.68 (0.14–3.19) | 0.632 | 3.10 (1.62–5.94) | 0.001** | 0.62 (0.20–1.90) | 0.409 |
| Gastroesophageal reflux | 0.56 (0.12–2.60) | 0.465 | 0.83 (0.41–1.67) | 0.609 | 0.74 (0.26–2.08) | 0.578 |
| Obstructive sleep apnea | 2.50 (0.59–10.48) | 0.210 | 0.98 (0.44–2.19) | 0.976 | 1.2 (0.37–3.81) | 0.758 |
| COPD | 0.43 (0.05–3.43) | 0.430 | 0.92 (0.40–2.08) | 0.846 | 4.25 (1.75–10.32) | 1.000 |
| Asthma | 1.40 (0.37–5.28) | 0.613 | 1.58 (0.77–3.25) | 0.208 | 0.88 (0.28–2.72) | 0.831 |
| Connective tissue disease | 1.08 (0.89–1.32) | 0.415 | 0.96 (0.84–1.09) | 0.562 | 0.96 (0.80–1.16) | 0.721 |
| Ischemic heart disease | 1.05 (0.22–4.92) | 0.949 | 2.58 (1.22–5.46) | 0.013 | 1.51 (0.52–4.33) | 0.439 |
| Stroke | 0.26 (0.03–2.10) | 0.211 | 3.10 (1.63–5.90) | 0.001** | 1.00 (0.38–2.62) | 1.000 |
| Osteoarthritis | 2 (0.59–6.70) | 0.261 | 0.70 (0.31–1.56) | 0.388 | 0.59 (0.16–2.07) | 0.415 |
| Interstitial lung disease | 0.95 (0.62–1.37) | 0.774 | 1.10 (0.88–1.56) | 0.635 | 4.85 (1.08–21.58) | 0.038* |
| Inflammatory bowel disease | 0.98 (0.73–1.12) | 0.654 | 3.72 (0.96–14.34) | 0.055 | 1.00 (0.42–2.31 | 1.000 |
COPD: chronic obstructive pulmonary disease; OR: odds ratio; CI: confidence interval.
Several findings arose from the present study. Firstly, patients with bronchiectasis showed a high rate of comorbidities. Secondly, these comorbidities were associated with poor outcomes in patients admitted for exacerbation. And last, bronchiectasis prognostic scores were not useful to predict outcomes in patients admitted for an exacerbation of bronchiectasis.
The most frequent group was cardiovascular comorbidities and some of them (diabetes mellitus, chronic heart failure and stroke) were associated with an increased hospital stay. Musculoskeletal comorbidities such as osteoporosis (31.6%) and osteoarthritis (19.6%) were also frequent comorbidities and were associated with an increased hospital stay. Regarding respiratory comorbidities, we detected obstructive sleep apnea (21%), COPD (16.9%) and asthma (15.1%), being somewhat less frequent than in other studies where asthma prevalence ranged between 20 and 30% and COPD 20–50%.6 This variability was probably because it is often difficult to know whether COPD or asthma are the cause of bronchiectasis or just comorbidities, given that a significant number of patients with bronchiectasis manifest persistent airway obstruction and could fit in the diagnosis of both COPD and asthma. In general, treatment objectives should be based on recommendations for both diseases.7 In our study, the coexistence of a respiratory condition with bronchiectasis seemed not to confer severity to these patients, as it did not increase the risk of mortality, increased hospital stay or readmission. Among other prognostic factors, we identified hypercapnia to be related with an increased in-hospital mortality. Both mMRC dyspnea scale and isolation of PA in the sputum during the hospitalization were associated with an increased hospital stay.
Several indexes such as BSI, FACED, E-FACED, and BACI have been developed and used as predictors of mortality and other outcomes in outpatients with bronchiectasis.2–4,8–12 Also, it has been demonstrated the relevance of respiratory and non-respiratory comorbidities in the prognosis of patients with bronchiectasis.4,5,7,8,13–17 In our study EFACED and BACI were associated with an increased hospital stay (p=0.002 and p=0.003, respectively), while Barthel index was associated with an increased risk of readmission 30 days after discharge (p=0.002). Although BSI had a good mortality predictive ability in this cohort with an AUC of 0.78, it did not reach statistical significance after adjusting for multiple comparisons. Unfortunately, none of the scores seemed to be good predictors of the outcomes in our cohort.
The specific treatment of comorbidities could benefit the management of patients with bronchiectasis.7 Implementation of clinical pathways for the management of other respiratory conditions such as COPD suggest that indicators like readmissions or length of stay, but not mortality, could be improved in those patients under certain interventions, when compared to those who are not included in the intervention.18 A multidisciplinary team of healthcare professionals during a hospital admission, together with a discharge plan, could probably have a beneficial effect on the clinical outcomes after an admission for an exacerbation of bronchiectasis.19
The main limitation of our study is that our patients were recruited from specialized bronchiectasis clinics representing a subset of patients with more advanced phases therefore our findings could not be generalizable for milder bronchiectasis populations.
In conclusion, patients with bronchiectasis showed a high rate of comorbidities, which are associated with poorer outcomes in patients admitted for exacerbation. Bronchiectasis prognostic scores are not useful to predict outcomes in patients admitted for an exacerbation of bronchiectasis. It would be interesting to create a predictive score for outcomes in bronchiectasis admissions given the clinical and economic relevance of these hospitalizations.
Authors’ contributionsStudy design: IGO, AR, JAC.
Data collecting: BUR, MC, CF.
Statistical analysis: IGO, JR.
Wrote the manuscript: IGO, BUR.
Read critically the manuscript: IGO, BUR, MC, CF, AR, JAC.
Data availability statementAll data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
Conflict of interestsNone.
Not applicable.