Smoking is a chronic addictive disease, and it is the world's main cause of preventable death and preventable disability. It is estimated that tobacco contributes to more than 8 million deaths each year worldwide.1,2
Smoking cessation is difficult to achieve since tobacco dependence involves behavioral, and cognitive phenomena such as attention, reward effect and memory. In fact, according to different studies: without help only 5% of smokers remain abstinent at 6 months, while medical treatment results in abstinence rates of 19–47% at 6 months.2,3
In this context, and knowing that not all smokers are the same, the question arises as to whether functional brain imaging could be a helpful tool as a neurobiomarker to achieve smoking cessation on the way to personalized medicine.3
Neural pathways and neuronal plasticity in nicotine dependenceThe brain is structurally and functionally modified by sustained exposure to nicotine. When a persone smokes, nicotine reaches the brain with 15s, dopaminergic neurons in the ventral tegmental area of the midbrain are activated, which increases dopamine in the nucleus accumbens.1 The acute brain response to the arrival of nicotine activates the prefrontal cortex (PFC), thalamus and the vision system. This fact produces a reinforcement of the behavior. Eventually there are changes in the output neurons of the nucleus accumbens and prefrontal cortex after sensitization. There is a remodeling of neuronal contacts and pathways, which is known as neuronal plasticity after sustained exposure to nicotine.1,2
Events that occur with acute brain damage in smokers, as occurs in a stroke, are an important source of information to know the nicotinic circuits involved in tobacco addiction and their operation, especially if after this brain damage the patient's addiction remits. A link between neuroanatomy and possible therapeutic benefit can thus be established. For example, the study by Naqvi et al. in 2007 showed that brain lesions that damage the insula are more likely to cease nicotine addiction without relapsing.4 However in 2018 the discovery of the connectome,5 a map of human brain connectivity, broke with the traditional neurological approach, demonstrating that in the case of addictions the relationship between lesion location and symptoms is not something so simple, but requires to integrated function of multiple brain regions with specific connectivity.
Recently in the study conducted by Joutsa et al. with a prospective cohort of 129 smokers suffering from focal brain damage, they found that a lesion likely to lead to nicotine addiction remission would be positively connected to the dorsal cingulate and insula but negatively connected to the medial prefrontal and temporal cortex.6 And what is also interesting is that the connectivity profiles of lesions disrupting nicotine addiction is similar to the connectivity profile of lesions reducing the risk of alcoholism, suggesting a shared network for addiction across the substances of abuse.6 In fact, other studies have shown that tobacco and alcohol dependence have a certain mutual predictive relationship and a common biological mechanism.7
Functional brain imaging as a biomarkerFunctional imaging techniques allow the simultaneous measurement of functional brain activity and behavior, which can be a valuable tool for understanding the brain structures and neurochemical pathways underlying craving and emotional, cognitive, motivational and reinforcing effects of the consumption of psychotropic substances.3 Many functional brain imaging studies of tobacco use and dependence have been performed: postitron emission tomography, single photon emission computed tomography and functional magnetic resonance imaging (fMRI). These techniques have seen a decrease in gray matter (GM) volume and/or density in smokers in multiple regions, including the prefrontal cortex, anterior cingulate, thalamus, temporal lobe and cerebellum.8,9 Previous studies revealed that thalamus participates in several cognitive brain functions, including inhibitory control, arousal regulation, sustained attention and others. Smaller thalamus volume may be related to the disruption of these cognitive function in smokers. With functional brain imaging it has also been seen that patients with nicotine dependence have reduced sensitivity to natural rewards (e.g. food, water, sex).3
Through fMRI good spatial resolution is achieved, with also good temporal resolution and does not require the administration of radiotracers. Also other studies with fMRI showed: reduced functional connectivity between the nucleus accumbens and insula at baseline was associated with poor smoking cessation outcomes after a 3-week quit attempt, decreased connectivity in smokers between the dorsolateral PFC and rostral anterior cingulate gyrus, and also decreased connectivity betweeen the anterior insula and PFC.8 In the study by Qian et al. they found that functional connectivity between the left dorsal medial thalamus and cerebellum is significantly decreased in the relapser group.9 In short, participants who relapsed had reduced functional connectivity between the conscious regions controlling the desire to smoke and those related to motor control.
As different people have differently wired brain circuits, the use of functional connectivity assessments as a tool for the characterization of addiction-related circuit alterations, consider this as a diagnostic tool to stratify individuals and potentially identify personalized treatments with higher probabilities of outcomes success.3
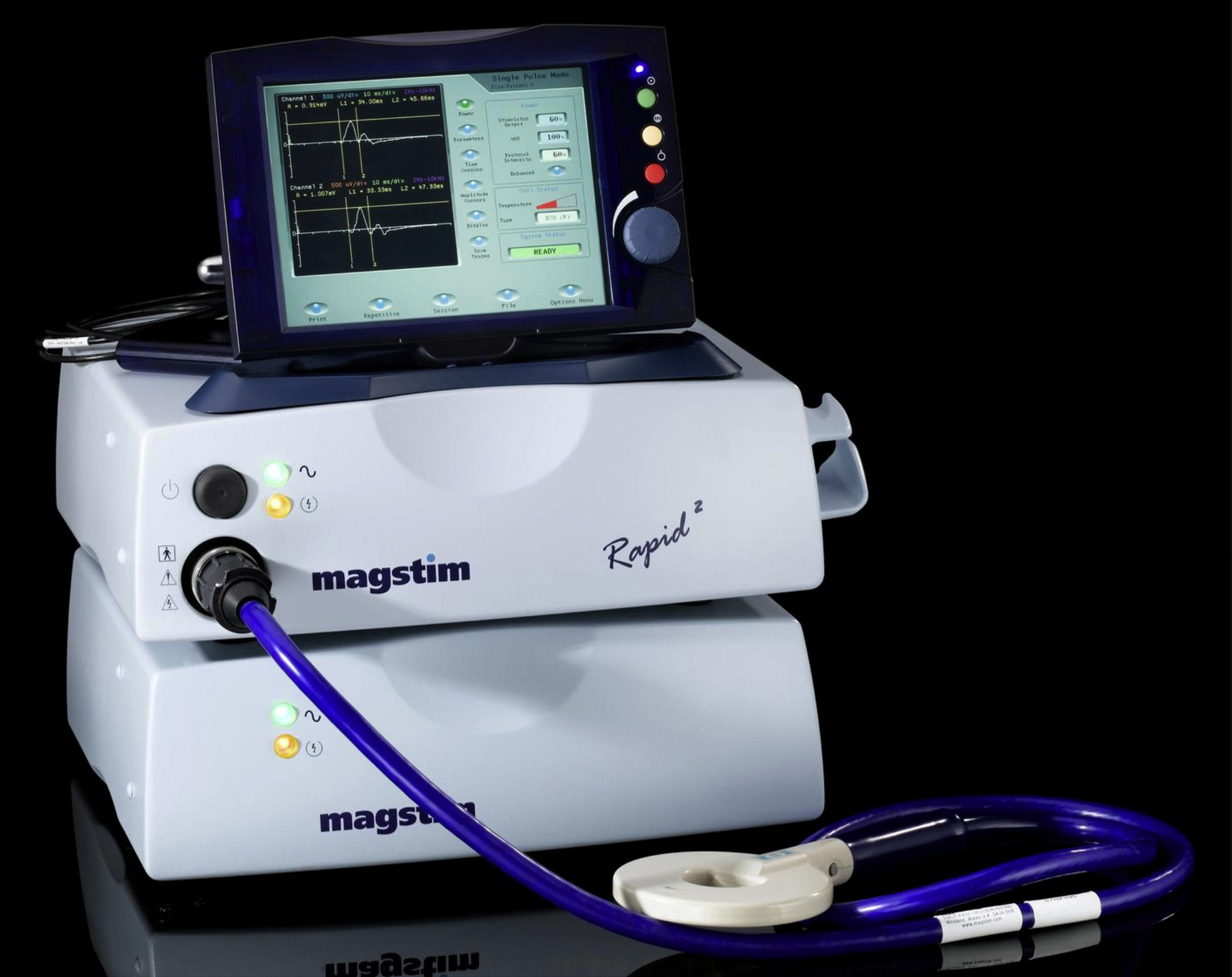
Next step: non-invasive brain stimulationNon-invasive brain stimulation (NIBS) methods such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) are promising treatments for nicotine dependence. These techniques have already been tried to treat other addictions such as alcoholism, as well as other psychiatric illnesses like depression or obsessive-compulsive disorder. In fact, in 2020 the Food and Drug Administration grant marketing approval10 to the Brainway deep TMS systems as an aid in short-term smoking cessation (Fig. 1). This approval was based on data from a randomized, double blind, sham controlled, multi-center trial of 262 chronic smokers who had made at least one prior failed attempt to quit, with the four-week continuous quit rate until week 18 was 19.4% following active and 8.7% following sham rTMS, verified by urine cotinine measures.11
NIBS can be diverse in modalities but also in stimulation parameters and montage. In rTMS, magnetic fields induce focal electrical currents indirectly and enable focal stimulations of the target area, more frequently with high-frequency (10Hz) in the PFC with cue provocation.12 The enhanced dorsolateral PFC activity improves executive function and cognitive control, and may increase dopamine release, and this can counterbalance the reward system and help patients cope with withdrawal and craving periods. On the other hand, tDCS involves the alteration of neuronal membrane polarization without triggering action potentials. It uses an H-coil that targets deeper (5–7cm) brain areas, such as the bilateral PFC and insula.13 The adverse effects described are few: headache, local pain, aesthenia, burning sensation, and as more serious cognitive disorder, syncope and epilepsy.
What appears clear in different meta-analyses of clinical trials is that multi-session protocols yielded larger effect sizes for reducing cravings and consumption than single-session protocols and that rTMS was therapeutically more effective than tDCS, possibly because rTMS can target the brain regions more-precisely than tDCS can.14,15 However, nowadays it remains unclear if these techniques can be combined with medical treatment for resistant smokers or it can be an alternative for patients who cannot tolerate medication side effects.14,15 Also if the duration of abstinence is maintained over the long term remains unclear.14,15 Large-scale randomized controlled trials with longer follow-up are needed.
Conflict of interestsThe authors state that they have no conflict of interests.