Indications for extracorporeal membrane oxygenation (ECMO), such as short-term life support in patients with severe respiratory or heart failure refractory to conventional treatment, have expanded considerably in the past 20 years.1 ECMO may be used in acute respiratory distress syndrome (ARDS) and other forms of potentially reversible respiratory failure as a bridge to recovery, definitive surgical intervention,2 or transplantation.3 The use of ECMO has also been described in the setting of serious respiratory infections, such as those caused by influenza A (H1N1) virus4 and, more recently, severe acute respiratory syndrome coronavirus type 2.5 However, scant data are available on its potential use in complications due to parasitic infections.6,7 We report the case of a patient with a diagnosis of bilateral pulmonary hydatid cysts undergoing antiparasitic treatment prior to surgery. One of the cysts ruptured, and the patient required vital support with ECMO as a bridge to definitive surgery.
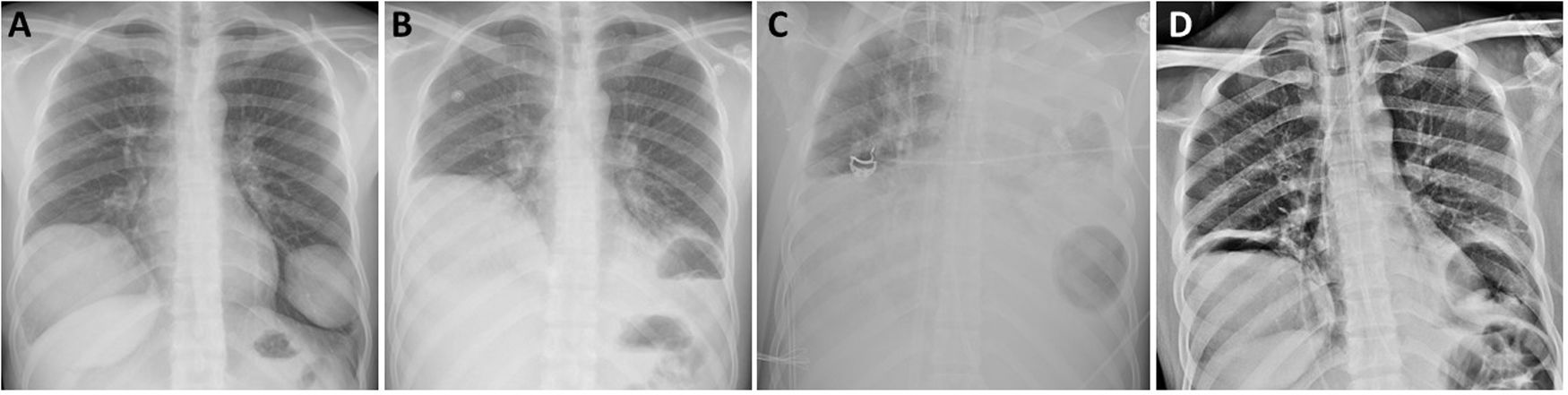
The patient was a 21-year-old Peruvian man, allergic to metamizole and diazepam, with no toxic habits or medical history of interest, who attended the emergency department with a 3-day history of cough and bloody expectoration, without any other accompanying symptoms. On chest X-ray, 2 masses with well-defined edges were observed in both lower lobes, along with signs of pulmonary hyperinflation (Fig. 1a). Chest computed tomography showed 2 pulmonary cystic lesions: one measuring 10.5 × 9.8 cm in the right lower lobe and the other measuring 6.2 × 6.1 cm in the left lower lobe. Radiological findings were suggestive of uncomplicated pulmonary hydatid cysts. Blood tests were positive for anti-Echinococcus granulosus antibodies, so we decided to start antiparasitic treatment with albendazole (400 mg/12 h) and praziquantel (1200 mg/12 h) prior to surgery. The patient’s lung function tests showed a restrictive ventilatory pattern.
Chest X-ray at diagnosis showing 2 uncomplicated cystic lesions (A); following rupture of the left lung hydatid cyst (B); air-fluid level after placement of ECMO, showing opacification of the entire left hemithorax and air in the left lung base (C); and after withdrawal of ventilatory support and ECMO (D).
While waiting for surgery, the patient spontaneously presented with pain in the left hemithorax and cough, followed by expectoration of blood and a small amount of pus. Physical examination of the patient revealed tachypnea (>25 rpm), tachycardia (>130 bpm), hypotension (103/57 mmHg), and SatO2 92% with oxygen supply via nasal prongs at 2 L per minute. The laboratory tests showed leukocytosis 11,420/μL, with 6.2% eosinophils and CRP of 4.36 mg/dL. Chest X-ray showed an air-fluid level in the basal region of the left hemithorax consistent with complicated hydatid cyst communicating with the airway (Fig.1B). Within a few hours, the patient presented generalized deterioration and acute respiratory failure and required admission to the intensive care unit (ICU) and urgent orotracheal intubation. Despite protective pulmonary ventilation, the patient worsened progressively with respiratory acidosis, hypercapnia 70 mmHg, Pao2/FiO2 < 100 mmHg, severe ventilation difficulty, and a tendency toward arterial hypotension. Given the persistence of the poor respiratory condition consistent with ARDS caused by rupture of the left hydatid cyst, we decided to administer support therapy with veno-venous ECMO (Fig. 1c). The patient received empirical antibiotic treatment with piperacillin/tazobactam and linezolid, and the microbiological cultures carried out ruled out bacterial superinfection. After hemodynamic and respiratory stabilization of the patient was achieved, his subsequent progress was satisfactory, with decreased ventilatory and ECMO support. ECMO was withdrawn completely 10 days after placement, and the patient was extubated 3 days later. He was discharged from the ICU to the hospital ward for rehabilitation treatment based on respiratory physiotherapy prior to the scheduled surgical procedure. One week later, surgical resection of both lung lesions was performed by bilateral thoracotomy without muscle section, consisting of right lower lobectomy and atypical resection of the left anterior-basal segment. During the postoperative period, the only complication was delirium with disorientation and altered level of consciousness, so an assessment by the neurology department was requested. Clinical and radiological evaluation with magnetic resonance imaging of the head established a diagnosis of diffuse microbleeds in a critically ill patient, probably associated with ECMO therapy, that did not require any specific treatment. The patient was discharged on postoperative day 7 after resolution of the neurological syndrome and referred to the infectious diseases department for monitoring of his medical treatment and follow-up.
ARDS associated or not associated with anaphylactic shock following rupture of a pulmonary hydatid cyst is very rare, although several cases have been described.6,8,9 However, management with ECMO has only been described in one case so far.6 In this setting, veno-venous ECMO support provides good oxygenation and ventilation, and helps minimize ventilatory support and the barotrauma associated with mechanical ventilation.10 However, ECMO therapy is not free of complications, the most frequent being those of a hemorrhagic or thrombotic nature.11
This case highlights the usefulness of ECMO as a bridge to definitive surgical treatment in the acute management of patients with ARDS due to rupture of a pulmonary hydatid cyst.
Please cite this article as: Gómez-Hernández MT, Martínez EJ, Fuentes MG, Paz M, Rodríguez I, Novoa NM, et al. Membrana de oxigenación extracorpórea (ECMO) como terapia puente a la cirugía en paciente con síndrome de distrés respiratorio agudo (SDRA) debido a la rotura de un quiste hidatídico pulmonar. Arch Bronconeumol. 2021;57:503–504.