Pleuroparenchymal fibroelastosis (PPFE), a rare interstitial lung disease, has recently been considered a type of idiopathic interstitial pneumonia in the European Respiratory Society/American Thoracic Society guidelines.1 Compared with idiopathic pulmonary fibrosis, PPFE has the following unique clinical, physiological, and histological characteristics2,3: low body mass index (BMI), flattened chest wall, markedly decreased forced vital capacity (FVC) with increased residual volume (RV)/total lung capacity (TLC) ratio,2 and fibrosis involving both the visceral pleura and subjacent subpleural lung parenchyma with upper lobe predominance.3 Although patients have been empirically treated with corticosteroids and several immunosuppressants, no treatments have been effective against PPFE.4,5 Lung transplantation can be considered another treatment option in advanced disease.4 Yanagiyama et al. reported a case of PPFE successfully treated with living donor bilateral lobar lung transplantation.6 They also emphasized the importance of pulmonary rehabilitation, which was required to reverse the flattened chest wall at posttransplantation.6
Pulmonary rehabilitation is a core aspect in the management of patients with chronic respiratory diseases.7 Providing tailored pulmonary rehabilitation requires better understanding of respiratory mechanics, particularly chest wall motion, in addition to ventilatory impairment, skeletal muscle function, cognitive function, and pulmonary gas exchange states.8 However, chest wall motion has not yet been clarified in patients with PPFE. This current report represents an experimental trial with dynamic breathing magnetic resonance imaging (MRI) to evaluate diaphragm and chest wall motions in patients with PPFE.
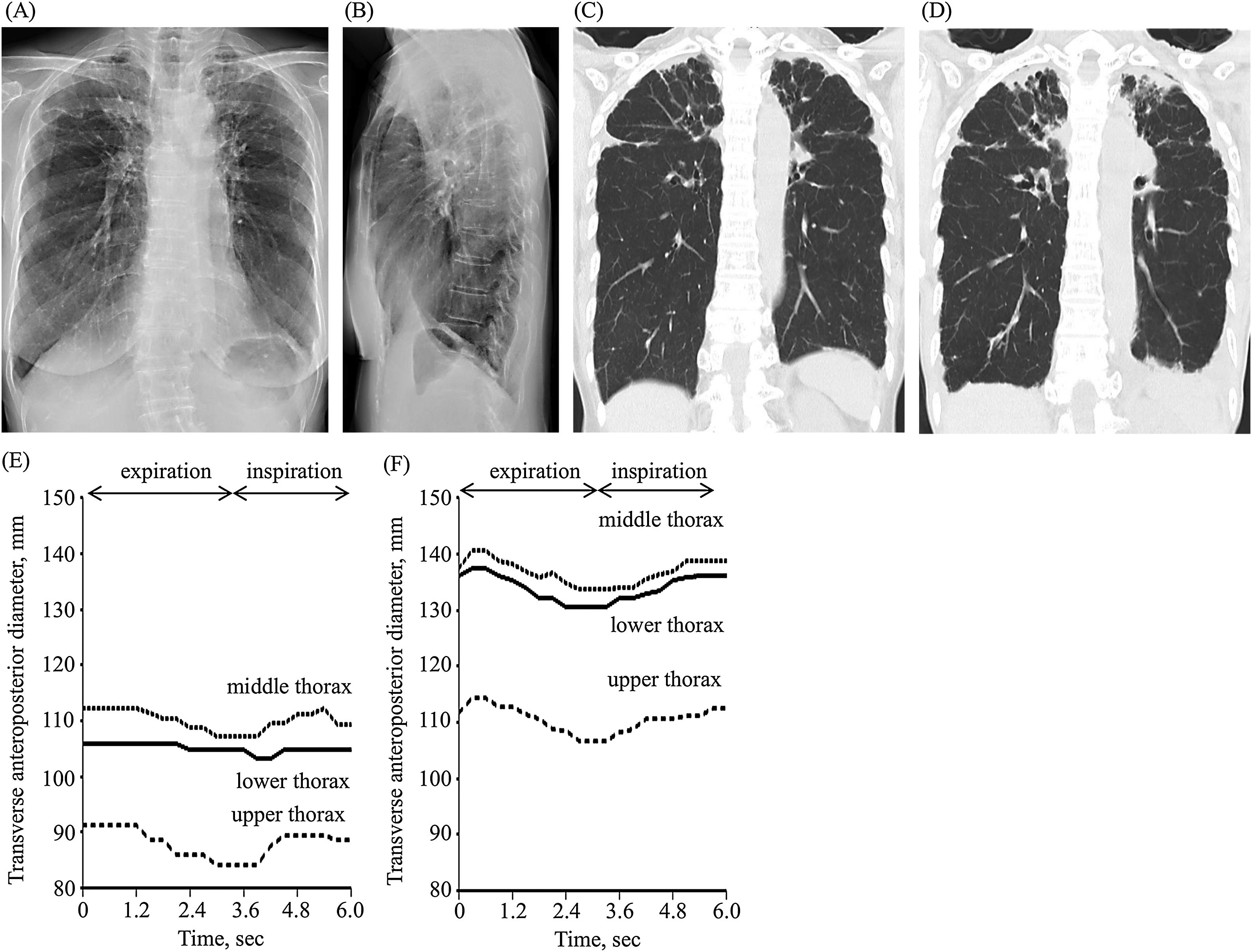
A 78-year-old nonsmoker woman with atrial tachycardia and cervical vertebral longitudinal ligament ossification was diagnosed with limited cutaneous systemic sclerosis and secondary Sjogren syndrome when she was 76. During diagnosis, her BMI was 17.3kg/m2. Chest X-ray showed upward shifting of both hilar areas and reticular shadow with apex opacities in both lungs in the frontal view (Fig. 1A) and chest flattening in the lateral view (Fig. 1B). Coronal chest computed tomography (CT) showed pleural thickening associated with subpleural fibrosis that was concentrated on both upper lobes (Fig. 1C). The percentage of predicted FVC and forced expiratory volume in one second (FEV1) were 59.9% (1.33L) and 70.9% (1.27L), respectively. She was diagnosed with radiological PPFE.3 At the age of 78, her BMI and the percentage of predicted FVC decreased to 13.6kg/m2 and 46.3% (1.08L), respectively. Coronal chest CT image showed the progression of pleural thickening with subpleural fibrosis and left-sided pleural effusion (Fig. 1D). She complained of progressive shortness of breathing that had gradually worsened during the previous year. Therefore, dynamic breathing MRI was performed to evaluate the diaphragm and chest wall motions during respiration. MRI was performed on a 1.5-T MAGNETOM Avanto (Siemens Healthcare GmbH, Erlangen, Germany) using a 16-channel body matrix coil and SR-Turbo-FLASH (echo time, 1.31ms; repetition time, 242ms; and flip angle, 12°). The field of view, matrix size, image slice, and acquisition time per image were 350mm, 160×160, 10mm, and 0.3s, respectively. Dynamic breathing MRI was performed at a fixed mid-sagittal plane through the middle of the right lung, while the patient was instructed to take repeated deep and smooth breaths from maximal inspiration to maximal expiration. The transverse anteroposterior diameter of images obtained by dynamic breathing MRI was evaluated at three fixed levels through the upper, middle, and lower thoraces using the ImageJ software (National Institutes of Health, Maryland, United States). Thereafter, dynamic breathing MRI was performed on a 41-year-old healthy male volunteer without any medical or tobacco use history. His BMI was 17.3kg/m2. The percentage of predicted FVC and FEV1 were 96.5% (4.26 L) and 94.7% (3.63 L), respectively. In the patient with PPFE (Supplemental video 1) and the healthy volunteer (Supplemental video 2), the diaphragm moved caudally and cranially during breathing. However, lower thoracic movement was decreased in the patient with PPFE compared with that in the healthy volunteer (Fig. 1E, F).
Frontal (A) and lateral (B) chest X-ray images at the age of 76. Coronal chest computed tomography images at the age of 76 (C) and 78 (D) years. Transverse anteroposterior diameter changes of images obtained by dynamic breathing magnetic resonance imaging in a patient with pleuroparenchymal fibroelastosis (E) and a healthy volunteer (F).
To the best of our knowledge, this is the first report describing the evidence of limited lower chest wall motion on dynamic breathing MRI in a patient radiologically diagnosed with PPFE, which could help to build up pulmonary rehabilitation suitable for PPFE. Sekine et al. previously described impaired lower chest wall motion in patients with unilateral upper pulmonary fibrosis post thoracotomy, which was radiologically consistent with PPFE but limited to one lung, according to dynamic breathing MRI.9 However, this chest wall motion impairment could be partially influenced by thoracotomy.
PPFE has unique physiological characteristics in that pulmonary function test reveals a markedly decreased FVC with increased RV/TLC ratio as described above.2 This restrictive pulmonary function pattern is generally found in patients with neuromuscular disorders and chest wall deformity such as scoliosis. Kotani et al. reported that the diaphragm motion was preserved but the chest wall motion was entirely limited using dynamic breathing MRI in patients with scoliosis.10 In contrast, in our case, lower chest wall motion was mainly limited, whereas the diaphragm motion was preserved. PPFE is characterized by pleural fibrosis and subpleural lung parenchymal fibroelastosis with upper lobe predominance and a flattened thoracic cage as described.3 These characteristics suggest that patients with PPFE have restricted wall motion in the upper or entire chest; however, this case showed conflicting chest wall motion characteristics. Unfortunately, the reason for this is unclear. Future studies with large numbers of patients with PPFE using dynamic breathing MRI as well as alternative methods such as optoelectronic plethysmography and four-dimensional chest CT are required to clarify chest wall motion characteristics and pathophysiological PPFE features and build up pulmonary rehabilitation suitable for PPFE.
FundingThis study was funded in part by the JSPS KAKENHI 19K17634 (SH). The Department of Advanced Medicine for Respiratory Failure is a Department of Collaborative Research Laboratory funded by Teijin Pharma, Japan.
Conflict of interestSatoshi Hamada and Tomohiro Handa report grants from Teijin Pharma, Japan, outside the submitted work.
We have no acknowledgements.