To evaluate the impact of the body position on primary central sleep apnea syndrome.
MethodsFifty-five subjects diagnosed with central sleep apnea (CSA) through polysomnographic examinations were prospectively enrolled in the study. All patients underwent cardiologic and neurologic examinations. Primary positional central sleep apnea (PCSA) was determined when the supine Apnea–Hypopnea Index (AHI) was greater than two times the non-supine AHI. The primary PCSA and non-PCSA groups were compared in terms of demographic characteristics, sleep parameters, and treatment approaches.
ResultsOverall, 39 subjects diagnosed with primary CSA were included in the study; 61.5% of the subjects had primary PCSA. There were no differences between the primary PCSA and non-PCSA groups regarding age, sex, body mass index (BMI), co-morbidities, and history of septoplasty. In terms of polysomnography parameters, AHI (P=.001), oxygen desaturation index (P=.002), the time spent under 88% saturation during sleep (P=.003), number of obstructive apnea (P=.011), mixed apnea (P=.009), and central apnea (P=.007) was lower in the primary PCSA group than in the non-PCSA group. Twenty-nine percent of the patients in the primary PCSA group were recommended position treatment and 71% were recommended positive airway pressure (PAP) therapy; all patients in the non-PCSA group were recommended PAP therapy.
ConclusionsOur results demonstrated that the rate of primary PCSA was high (61.5%) and primary PCSA was associated with milder disease severity compared with non-PCSA. The classification of patients with primary CSA regarding positional dependency may be helpful in terms of developing clinical approaches and treatment recommendations.
Evaluar el impacto de la posición del cuerpo en el síndrome de apnea central del sueño primaria.
MétodosEn el estudio se incluyeron prospectivamente 55 sujetos con diagnóstico de apnea central del sueño (ACS) a través de sus estudios polisomnográficos. Todos los pacientes fueron sometidos a exámenes cardiológicos y neurológicos. La apnea central del sueño posicional (ACSP) se estableció cuando el índice de apnea-hipopnea (IAH) en posición supina fue más de dos veces mayor que el IAH en posición no supina. Se compararon los grupos de pacientes con ACSP primaria y sin ACSP en función de las características demográficas, los parámetros del sueño y los enfoques de tratamiento.
ResultadosEn total, se incluyeron en el estudio 39 sujetos con diagnóstico de ACS primaria. El 61,5% de los sujetos presentaban ACSP primaria. No hubo diferencias entre los grupos de ACSP primaria y sin ACSP con respecto a la edad, el sexo, el índice de masa corporal (IMC), las comorbilidades y los antecedentes de septoplastia. En cuanto a los parámetros de polisomnografía, el IAH (p=0,001), el índice de desaturación de oxígeno (p=0,002), el tiempo transcurrido con una saturación por debajo del 88% durante el sueño (p=0,003) y el número de apneas obstructivas (p=0,011), de apneas mixtas (p=0,009) y de apneas centrales (p=0,007) fueron menores en el grupo con ACSP primaria que en el grupo sin ACSP. Al 29% de los pacientes en el grupo de ACSP primaria se les recomendó tratamiento posicional y al 71% se les recomendó tratamiento de presión positiva de la vía aérea (PAP); a todos los pacientes del grupo sin ACSP se les aconsejó tratamiento PAP.
ConclusionesNuestros resultados demostraron que la tasa de ACSP primaria fue alta (61,5%) y la ACSP primaria se asoció con una enfermedad más leve en comparación con la ACS no posicional. La clasificación de pacientes con ACS primaria dependiente de la posición puede ser útil a la hora de desarrollar enfoques clínicos y recomendaciones de tratamiento.
Central sleep apnea syndrome (CSAS) is a clinical entity characterized by the temporary absence or diminution of respiratory drive coming from the respiratory center during sleep.1 Central sleep apnea (CSA) occurs in less than 5% of subjects admitted to sleep clinics.2 CSA could be primary (idiopathic) or secondary to medical conditions (e.g. heart failure, renal failure, cerebrovascular disease), high altitude, opioids, and drug use.3 Primary CSA is diagnosed by exclusion of any identifiable cardiac and neurologic cause in patients with CSA. Primary CSA seems to be driven by elevated chemosensitivity to PCO2. Circulation delay is defined as normal in these patients and is unlikely to contribute to CSA.4
Obstructive sleep apnea (OSA) is a more common and well-described form of sleep-disordered breathing (SDB) than CSA. One of the most striking features of OSA is that the respiratory events are particularly severe and frequent in the supine sleeping position.5 Indeed, supine-related OSA is a dominant phenotype of OSA with a prevalence of 20%–60% in the general population.6 Similar to OSA, in patients with medical conditions such as heart failure and stroke, the severity of CSA is increased when sleeping in the supine position compared with the lateral position.7,8 The possible reasons for supine-related worsening of central apneas in patients with medical conditions include upper airway and lung volume effects, and altering plant gain when in the supine position.9 Although the impact of sleeping position on patients with OSA and CSA with HF and stroke is well described, the effects of body position on primary CSA have not been commonly investigated. There are few reports in the literature of positional CSA in patients without cardiac history or congestive heart failure.10,11 Currently, phenotyping of SDB is widely discussed, but the lack of clinical studies considering primary CSA and positional dependence is evident.
In view of these findings, the purpose of the current study was to evaluate the impact of body position on primary central sleep apnea syndrome. Thereby, we also assessed the features of primary positional central sleep apnea (PCSA) and phenotypic approach to primary CSA.
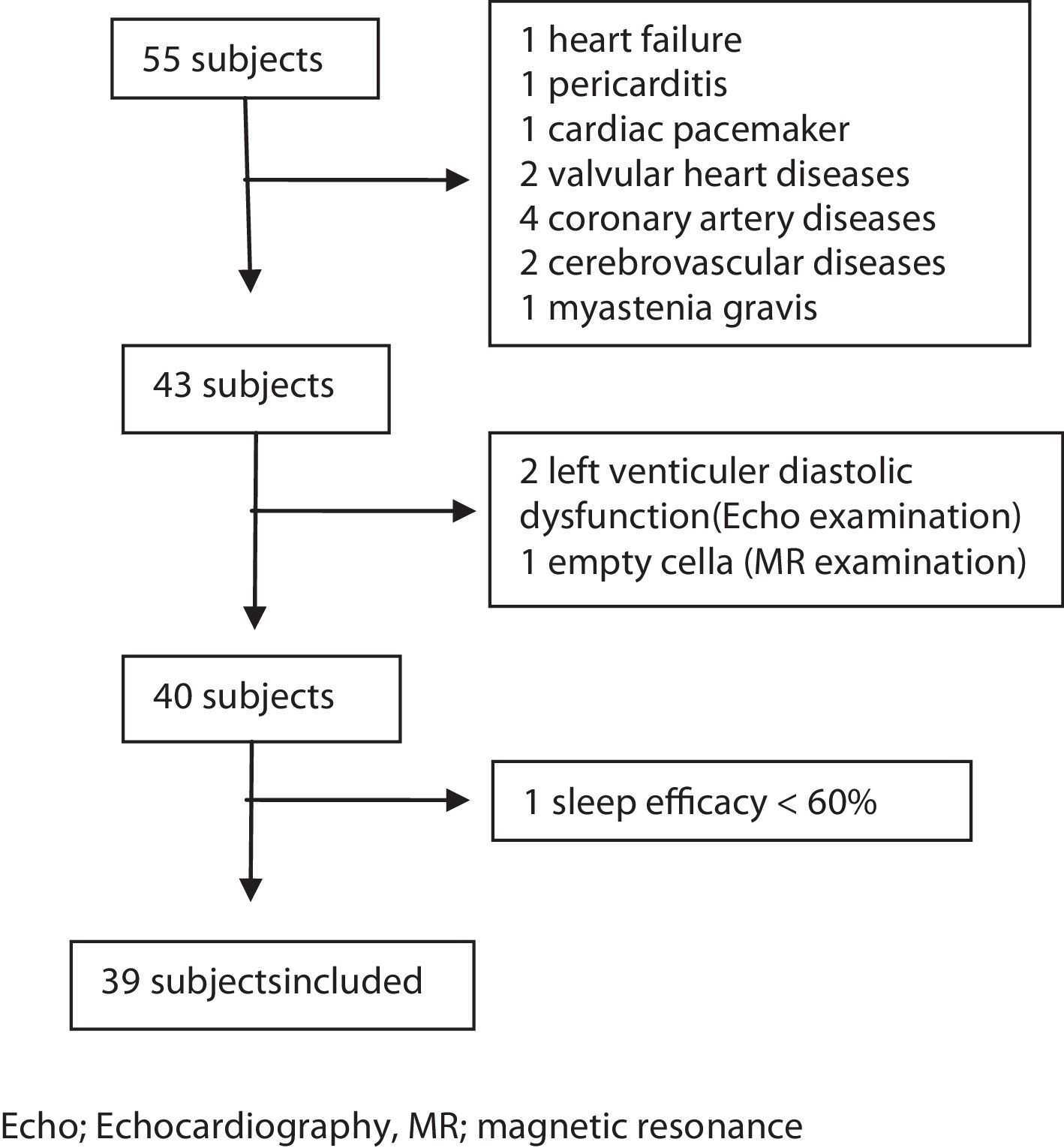
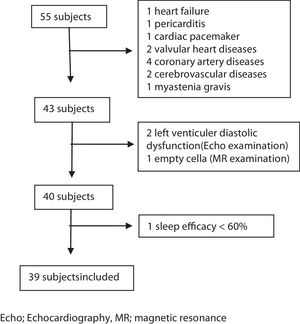
MethodsSubjectsThis study was a prospective study. Consecutive patients admitted to our sleep laboratory between June 1st, 2016 and June 1st, 2017, and diagnosed as having CSA with full-night polysomnography (PSG) examinations were enrolled in the study. During this period 10.326 patients admitted to our sleep laboratory and 1087 of those underwent PSG examination. Fifty-five patients were diagnosed as having CSA within this period. The criteria for inclusion were as follows: (1) age over 18 years; (2) no previous diagnosis and treatment of SDB; (3) presence of at least one of the following symptoms: a. Sleepiness. b. Difficulty initiating or maintaining sleep, frequent awakenings, or nonrestorative sleep. c. Awakening short of breath. d. Snoring. e. Witnessed apneas; (4) diagnosis of CSA syndrome with full-night PSG examination; (5) sleep efficacy ≥60%; (6) supine or non-supine sleeping time more than 30min; (7) completed anthropometric data, Epworth Sleepiness Scale (ESS), and STOP-Bang questionnaire. All patients underwent otolaryngologic examination, neurologic and cardiac evaluations. Magnetic resonance imaging (MRI) of the brain, and electrocardiographic and echocardiographic examinations were performed. Patients who were diagnosed as having any cardiac and neurologic disease or those with a history of previous cardiac and neurologic diseases were excluded from the study. Arterial blood gas was taken from the patients with a BMI 30 and above to rule out the obesity hypoventilation syndrome. The other exclusion criteria were as follows: diagnosis of other sleep disorders (e.g. insomnia, sleep-related movement disorders), upper airway pathology requiring surgery, active psychiatric disorders, history of renal failure, and opioid use (Fig. 1).
EchocardiographyEchocardiographic examination was performed by an experienced cardiologist using a Philips iE33×MATRIX device while the patient was in the left decubitus position.
Magnetic resonance: MR examinations were performed using a 1.5T MR scanner (Siemens, GE).
In-laboratory PolysomnographyThe diagnosis of CSA was made using in-lab PSG examinations. Electroencephalography, electro-oculography, and electromyography of the chin and leg (anterior tibialis), electrocardiography, oxygen saturation (from the fingertips), respiratory effort (thoracic, abdominal), and air flow (nasal pressure transducer and oronasal thermistor), body position, and tracheal was recorded with a Comet Grass Telefactor, version 4.5.3. PSG recordings were analyzed by a physician experienced in sleep disorders using the TWin® EEG/PSG Software. Scoring of sleep and respiratory events was performed according to the criteria of the American Academy of Sleep Medicine (AASM) manual, version 2.3.12 CSA was diagnosed as central Apnea–Hypopnea Index (AHI) ≥5 and the number of central apneas and/or central hypopneas was >50% of the total number of apneas and hypopneas. For the diagnosis of primary CSA another current sleep disorders, medical or neurologic disorders, medication and substance use were excluded.3 Diagnosis of positional CSA was determined when the supine AHI was greater than twice the non-supine AHI. The severity of CSA was categorized as follows: mild (5≤AHI<15events/h), moderate (15≤AHI<30events/h), and severe (AHI≥30events/h).
Treatment ApproachPatients with non-PCSA underwent positive airway pressure titration. If the patients were nonresponsive to continuous positive airway pressure (CPAP), bilevel positive airway pressure-spontaneous timed (BPAP-ST) and adaptive servo ventilation (ASV) was used, respectively.13 PAP titrations were performed based on the AASM guideline for adults.14
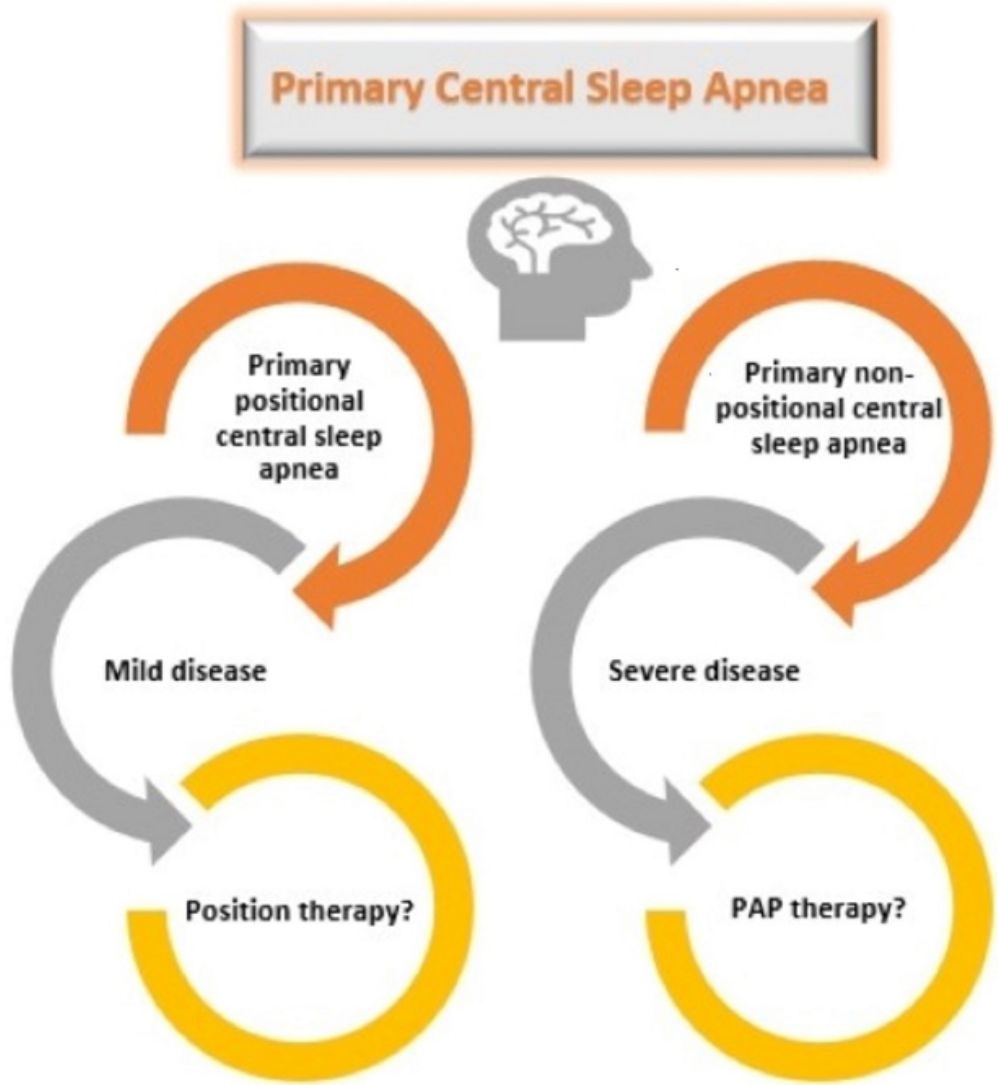
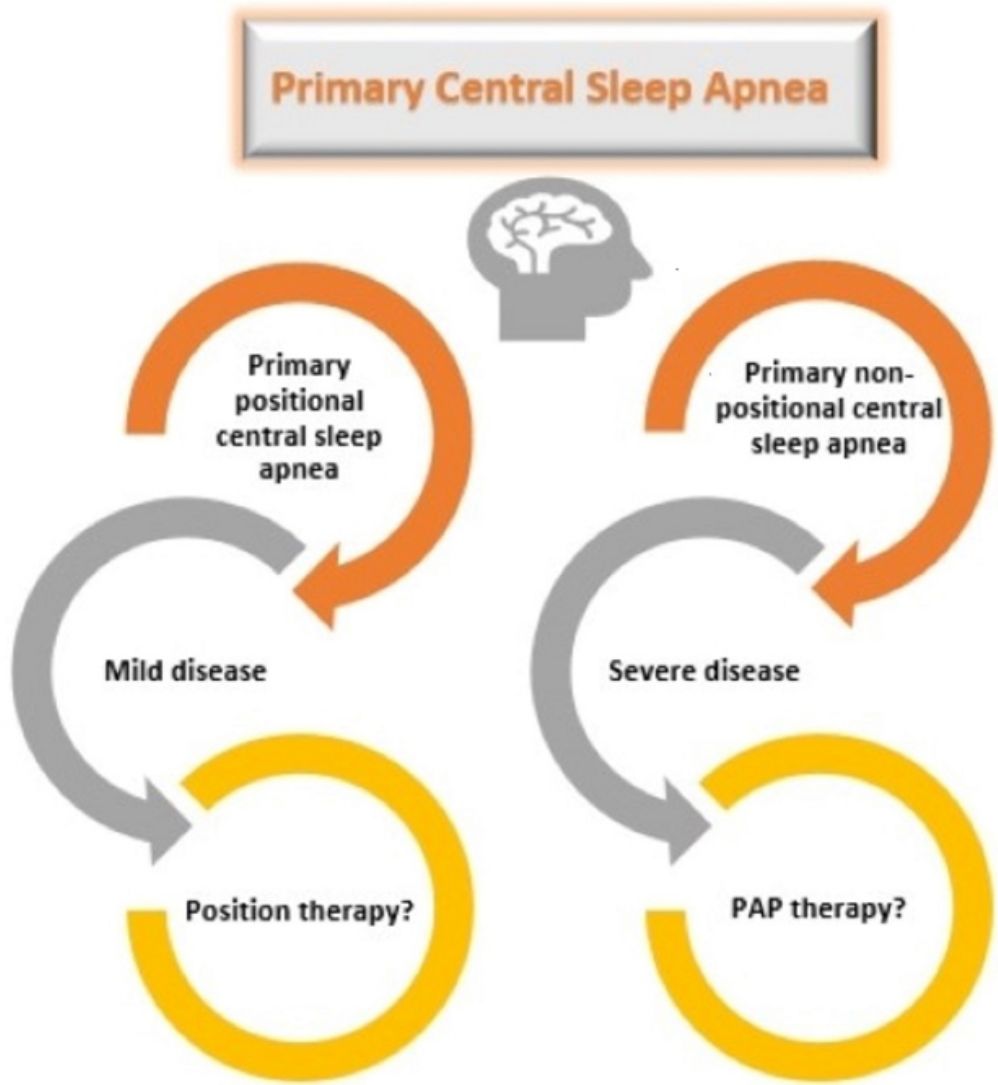
Patients with primary PCSA were further classified into two groups as supine-predominant (AHI≥5/h and supine AHI/non-supine AHI≥2) and supine-isolated (AHI≥5/h, supine AHI/non-supine AHI≥2, and non-supine AHI<5) as proposed by Joosten et al., before determining the therapeutic approaches.5 Position therapy was recommended to patients with supine-isolated primary PCSA. Patients with supine-predominant primary PCSA and patients who did not accept the position therapy in the supine-isolated group underwent PAP titration.
The study was approved by the local research ethics committee (Dr. Suat Seren Chest Disease and Chest Surgery, Training and Research Hospital, Date: 18.01.2016, Number: 572). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Statistical AnalysisQuantitative data are reported as mean±standard deviation (SD) or as median with minimum–maximum values, and qualitative data are reported as observed frequencies and percentages. The Shapiro–Wilk test was used to check normality and, according to the results, parametric or non-parametric suitable statistical tests were performed. An independent samples t-test or non-parametric alternative of the Mann–Whitney U test was used to compare two groups for a quantitative variable. The Chi-square test was used to assess associations between qualitative variables. All statistical analyses were performed using a statistics software package (SPSS Inc., version 25.0, Chicago, IL, USA) and the level of significance was set at 0.05.
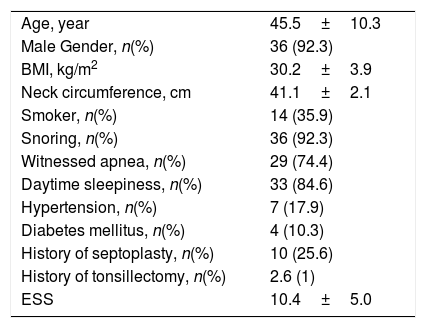
ResultsOverall, 39 subjects who were diagnosed as having primary CSA were included in the study. A total of 61.5% of the subjects had the diagnosis of primary PCSA. 92.3% of the study population were males and the 7.7% were females. The mean age of the subjects was 45.5±10.3 years and the mean body mass index (BMI) was 30.2±3.9kg/m2. 17.9% of the subjects had hypertension (HT), 10.3% had diabetes mellitus (DM), and 25.6% had a history of septoplasty surgery. Only one subject had previously undergone tonsillectomy. Regarding sleep symptoms; 24 patients had the symptoms of snoring, witnessed apnea and daytime sleepiness, 11 patients had two of three symptoms, and four patients had only one symptom. The demographic characteristics of the study population are presented in Table 1.
Demographic Data of the Study Population (n=39).
| Age, year | 45.5±10.3 |
| Male Gender, n(%) | 36 (92.3) |
| BMI, kg/m2 | 30.2±3.9 |
| Neck circumference, cm | 41.1±2.1 |
| Smoker, n(%) | 14 (35.9) |
| Snoring, n(%) | 36 (92.3) |
| Witnessed apnea, n(%) | 29 (74.4) |
| Daytime sleepiness, n(%) | 33 (84.6) |
| Hypertension, n(%) | 7 (17.9) |
| Diabetes mellitus, n(%) | 4 (10.3) |
| History of septoplasty, n(%) | 10 (25.6) |
| History of tonsillectomy, n(%) | 2.6 (1) |
| ESS | 10.4±5.0 |
BMI, body mass index; ESS, Epworth Sleepiness Scale.
Data is depicted as mean±SD or number (percentage).
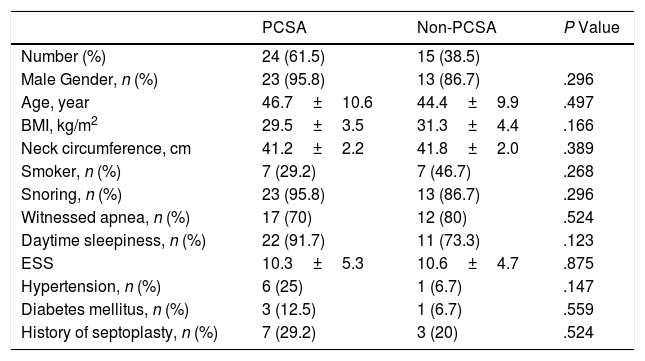
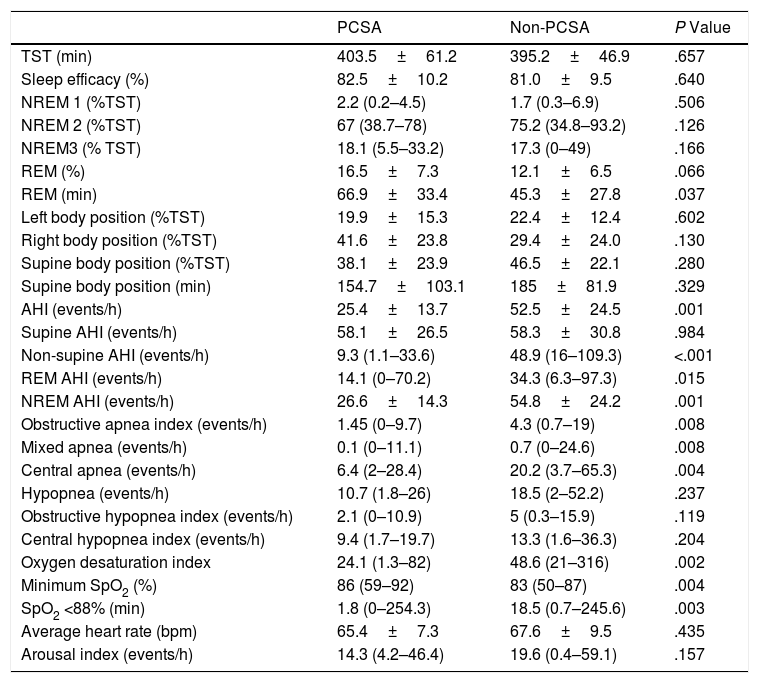
There were no differences between the primary PCSA and non-PCSA groups regarding age, sex, BMI, ESS, and history of HT, DM, and septoplasty (Table 2). In terms of PSG parameters; AHI (P=.001), rapid eye movement (REM) AHI (P=.015), non-REM AHI (P=.001), oxygen desaturation index (P=.002), the time spent under 88% saturation during sleep (P=.003), and the index of obstructive apnea (P=.008), mixed apnea (P=.008) and central apnea (P=.004) was lower in the primary PCSA group than in the non-PCSA group. The duration of REM sleep was longer in the primary PCSA group compared with the non-PCSA group (Table 3). There was no difference between the two groups in terms of supine AHI, but there was a statistically significant difference in terms of non-supine AHI (P=.984, P<.001, respectively).
Comparison of the Baseline Characteristics of the Primary PCSA and Non-PCSA Groups.
| PCSA | Non-PCSA | P Value | |
|---|---|---|---|
| Number (%) | 24 (61.5) | 15 (38.5) | |
| Male Gender, n (%) | 23 (95.8) | 13 (86.7) | .296 |
| Age, year | 46.7±10.6 | 44.4±9.9 | .497 |
| BMI, kg/m2 | 29.5±3.5 | 31.3±4.4 | .166 |
| Neck circumference, cm | 41.2±2.2 | 41.8±2.0 | .389 |
| Smoker, n (%) | 7 (29.2) | 7 (46.7) | .268 |
| Snoring, n (%) | 23 (95.8) | 13 (86.7) | .296 |
| Witnessed apnea, n (%) | 17 (70) | 12 (80) | .524 |
| Daytime sleepiness, n (%) | 22 (91.7) | 11 (73.3) | .123 |
| ESS | 10.3±5.3 | 10.6±4.7 | .875 |
| Hypertension, n (%) | 6 (25) | 1 (6.7) | .147 |
| Diabetes mellitus, n (%) | 3 (12.5) | 1 (6.7) | .559 |
| History of septoplasty, n (%) | 7 (29.2) | 3 (20) | .524 |
Data is depicted as mean±SD or number (percentage). BMI, body mass index; ESS, Epworth Sleepiness Scale.
Comparison of the Sleep Study Data of the Primary PCSA and Non-PCSA Groups.
| PCSA | Non-PCSA | P Value | |
|---|---|---|---|
| TST (min) | 403.5±61.2 | 395.2±46.9 | .657 |
| Sleep efficacy (%) | 82.5±10.2 | 81.0±9.5 | .640 |
| NREM 1 (%TST) | 2.2 (0.2–4.5) | 1.7 (0.3–6.9) | .506 |
| NREM 2 (%TST) | 67 (38.7–78) | 75.2 (34.8–93.2) | .126 |
| NREM3 (% TST) | 18.1 (5.5–33.2) | 17.3 (0–49) | .166 |
| REM (%) | 16.5±7.3 | 12.1±6.5 | .066 |
| REM (min) | 66.9±33.4 | 45.3±27.8 | .037 |
| Left body position (%TST) | 19.9±15.3 | 22.4±12.4 | .602 |
| Right body position (%TST) | 41.6±23.8 | 29.4±24.0 | .130 |
| Supine body position (%TST) | 38.1±23.9 | 46.5±22.1 | .280 |
| Supine body position (min) | 154.7±103.1 | 185±81.9 | .329 |
| AHI (events/h) | 25.4±13.7 | 52.5±24.5 | .001 |
| Supine AHI (events/h) | 58.1±26.5 | 58.3±30.8 | .984 |
| Non-supine AHI (events/h) | 9.3 (1.1–33.6) | 48.9 (16–109.3) | <.001 |
| REM AHI (events/h) | 14.1 (0–70.2) | 34.3 (6.3–97.3) | .015 |
| NREM AHI (events/h) | 26.6±14.3 | 54.8±24.2 | .001 |
| Obstructive apnea index (events/h) | 1.45 (0–9.7) | 4.3 (0.7–19) | .008 |
| Mixed apnea (events/h) | 0.1 (0–11.1) | 0.7 (0–24.6) | .008 |
| Central apnea (events/h) | 6.4 (2–28.4) | 20.2 (3.7–65.3) | .004 |
| Hypopnea (events/h) | 10.7 (1.8–26) | 18.5 (2–52.2) | .237 |
| Obstructive hypopnea index (events/h) | 2.1 (0–10.9) | 5 (0.3–15.9) | .119 |
| Central hypopnea index (events/h) | 9.4 (1.7–19.7) | 13.3 (1.6–36.3) | .204 |
| Oxygen desaturation index | 24.1 (1.3–82) | 48.6 (21–316) | .002 |
| Minimum SpO2 (%) | 86 (59–92) | 83 (50–87) | .004 |
| SpO2 <88% (min) | 1.8 (0–254.3) | 18.5 (0.7–245.6) | .003 |
| Average heart rate (bpm) | 65.4±7.3 | 67.6±9.5 | .435 |
| Arousal index (events/h) | 14.3 (4.2–46.4) | 19.6 (0.4–59.1) | .157 |
TST, total sleep time; Non-REM, non-rapid eye movement; REM, repeat eye movement; AHI, apnea–hypopnea index; SpO2, oxygen desaturation.
Interval data are expressed as mean±SD for normally distributed data, median (min–max) for non-normally distributed data.
According to the PSG results, six patients (15.6%) had mild CSA, 14 (35.9%) had moderate CSA, and 19 patients (48.7%) had severe CSA. When we compared the mild-to-moderate CSA group with the severe CSA group, a statistically significant difference was found regarding the history of septoplasty surgery (P=.022); two (10%) patients had a history of septoplasty in the mild-to-moderate group compared with eight (42.1%) in the severe CSA group.
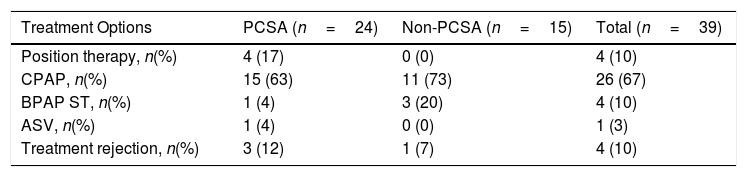
When the primary PCSA group was classified into two groups as supine-predominant and supine-isolated, 17 (71%) patients were in the supine-predominant group and 7 (29%) were in the supine-isolated group. The treatment methods applied to the subjects are illustrated in Table 4. Three of the seven patients who were recommended position therapy in the primary PCSA group could not adapt to the treatment. Therefore, PAP titration was also applied to these patients. Overall, 20 patients in the primary PCSA group were recommended PAP therapy, but three of those rejected PAP titration. Consequently 17% patients were applied position therapy and 71% received PAP titration (63% CPAP, 4% BPAP-ST, 4% ASV). In the non-PCSA group, PAP therapy was recommended to all patients, but one patient refused the therapy. The remaining 93% of patients in the non-PCSA group were responsive to PAP therapy (73% CPAP, 20% BPAP-ST). The mean CPAP pressure used for the responsive patients undergoing CPAP titration in the primary PCSA group and non-PCSA group was 8.00±2.44cm H2O and 8.36±1.91cm H2O, respectively.
Treatment Modalities Applied to the Subjects.
| Treatment Options | PCSA (n=24) | Non-PCSA (n=15) | Total (n=39) |
|---|---|---|---|
| Position therapy, n(%) | 4 (17) | 0 (0) | 4 (10) |
| CPAP, n(%) | 15 (63) | 11 (73) | 26 (67) |
| BPAP ST, n(%) | 1 (4) | 3 (20) | 4 (10) |
| ASV, n(%) | 1 (4) | 0 (0) | 1 (3) |
| Treatment rejection, n(%) | 3 (12) | 1 (7) | 4 (10) |
CPAP, continuous positive airway pressure; BPAP-ST, bi-level positive airway pressure-spontaneous/time mode; ASV, adaptive servo-ventilation.
In this study, we aimed to investigate the impact of body position on primary central sleep apnea syndrome. Our results demonstrated that primary PCSA was observed in 61.5% of patients with primary CSA. Additionally, primary PCSA was associated with milder disease severity compared with non-PCSA.
To our knowledge, this is the first study to evaluate the impact of body position on primary CSA syndrome and the features of primary PCSA. There are a limited number of studies on the relation between CSA and position in the literature. In these studies, the effect of body position in patients with CSA associated with hearth failure was evaluated.15,16 There are no prospective studies evaluating the relation between body position and primary CSA. Our results demonstrated that the primary PCSA ratio was high, similar to supine-related OSA. Pinna et al. reported that the severity of CSA decreased when the subjects were in the lateral position compared with the supine position in patients with congestive heart failure (CHF).15 Similar results were revealed in other studies.8,17 Several mechanisms have been proposed to explain the supine-related worsening of CSA in patients with CHF. Circulatory delay between the lungs and chemoreceptors was believed to play an important role in the pathogenesis of CSA in patients with CHF.4 However, the study conducted by Traversi et al. showed that the reduction of CSA severity from the supine position to the lateral position in patients with CHF was not due to the improvement in cardiac hemodynamics. They put forward that non-cardiac factors such as the change in functional residual capacity were likely to represent the main cause.16 Sleeping in the supine position can result in a reduction of both functional residual capacity and metabolic rate, which consequently enhances plant gain. Enhanced plant gain is defined as a large change in carbon dioxide levels relative to a small change in ventilation.11 The mechanism responsible for supine-related OSA is mainly related to the direct and indirect effects of gravity on upper airway collapsibility. Increased loop gain may also contribute to the pathogenesis of OSA.15 Reports on primary PCSA are rare as previously mentioned and the reason for positional dependency of primary CSA is not yet understood. The reduction in the functional residual capacity resulting in enhanced plant gain may also play a role in encouraging central apneas in patients with primary PCSA. Previous studies suggest that arousals play an important role in triggering and perpetuating central apneas in both HF and non-HF patients with CSA. Arousals can provoke a fall in PaCO2 below the apnea threshold that can result in central apnea.18 In this study there was no difference between primary PSCA and non-PCSA groups with regard to arousals index.
Primary PCSA is associated with milder disease severity, like positional OSA in this study. AHI, REM AHI, non-REM AHI, oxygen desaturation index, and the desaturation time spent under 88% during sleep were lower in patients with primary PCSA compared with those with non-PCSA. The duration of REM sleep was longer in patients with primary PCSA. Over the past decade, evidence for an association between REM sleep and neurologic and cardiovascular diseases has accumulated. Zhang et al. demonstrated that the proportion and duration of REM sleep were negatively associated with all-cause mortality.19 Primary PCSA is both associated with milder disease severity and longer REM sleep time compared with non-PCSA. These findings suggest that there may be a difference between primary PCSA and non-PCSA groups with regard to the risk of developing cardiometabolic complications. Hence, primary PCSA should be evaluated as a separate clinical entity.
The outstanding features of the patients with primary CSA in our study were their younger age, male predominance, and the presence of septoplasty history. Although there was no statistically significant difference, it was noteworthy that the presence of septoplasty history was higher in the primary PCSA group than in the non-PCSA group. Additionally, the history of septoplasty in patients with severe primary CSA was higher than in the mild-to-moderate group. There may be a link between nasal physiology and the development of CSA, which should be evaluated. Tanaka et al. proposed that after a switch to oral breathing during sleep, central respiratory events increase due to greater CO2 elimination during expiration.20 Nasal receptors responsive to air flow may be important in maintaining the rhythmicity of breathing and preventing airway collapse during sleep.21
Primary CSA has no proven standardized treatment. PAP therapy, use of acetazolamide, zolpidem, and triazolam are the treatment options for primary CSA; however, these drugs have limited supporting evidence.13 In this study, patients with primary PCSA were recommended PAP therapy as well as position treatment options. Position treatment was recommended principally to patients with supine isolated-PCSA. Four of these seven patients accepted position therapy as a treatment option. In patients with primary PCSA, 71% of the patients underwent PAP titration and 63% were responsive to CPAP. Unfortunately, the adherence of the patients with sleep apnea to the CPAP treatment was not sufficient.22 In clinical practice, positional therapy might be an alternative treatment in patients with primary PCSA. However, future research is required to validate this approach. PAP therapy was recommended to all patients in the non-PCSA group. The mean CPAP pressure was lower in the primary PCSA group than in the non-PCSA group.
Our study has some limitations that must be addressed. The number of patient population was too small to allow for subgroup analysis. However, it should be considered that the prevalence of primary CSA is low in the general population. Our hospital is a chest disease and chest surgery branch hospital. It is not a primary center for patients with serious cardiovascular diseases such as heart failure, renal failure, and cerebrovascular accidents. Therefore, rates of secondary CSA may be lower than those of primary CSA found in this study. Another major limitation of our study is that we could not measure the PC02 during sleep. We could only measure the PC02 values of the patients with a BMI 30 and above to rule out the obesity hypoventilation syndrome. PC02 values of 19 patients in whom BMI≥30kg/m2, was 38.2±1.20. None of the patients had the diagnosis of obesity hypoventilation syndrome. Complex sleep apnea syndrome (CompSAS) may be considered in patients with a history of septoplasty. The International Classification of Sleep Disorders (ICSD)-3 mentioned a case report of the development of central apnea following nasal surgery in a patient with known obstructive apnea.12 In this case, a PSG examination was performed 4 months after nasal surgery. Changes in carbon dioxide regulation have been implicated in the pathogenesis. It is proposed that central sleep apnea resolves in these patients over time, as seen in the vast majority of patients with CPAP-emergent central sleep apnea.23 Spontaneous resolution is reported in 50%–75% of PAP-emergent central apneas after several months of PAP therapy.24 Morgenthaler et al. showed that CompSAS resolved in about one-third to two-thirds of patients treated with CPAP after 90 days of therapy.25 In our study, history of septoplasty was obtained from the patient's own reports and was performed prior to admission to our clinic. None of the patients had undergone PSG examinations before surgery. There were 3.3±1.15 years between the nasal surgery and PSG examination. ICSD-3 also emphasized that treatment induced central apnea resolves with time, but the time course for this resolution is unclear. Longitudinal observation of the patients in our study could not be evaluated because it was not included in the study protocol.
ConclusionIt is important to reveal the relationship between primary CSA and body position. In this study, it was demonstrated that the rate of primary PCSA was high (61.5%) and primary PCSA was associated with milder disease severity. The classification of patients with primary CSA regarding positional dependency may be helpful in terms of developing clinical approaches and treatment recommendations. Further clinical studies are required to validate this approach.
Conflict of InterestThe authors declare that they have no conflict of interest.