The role of bronchial provocation tests in the diagnosis of asthma remains to be fully explored. We aimed to evaluate methacholine and mannitol challenge testing, and explore the factors associated with this broncoprovocation response.
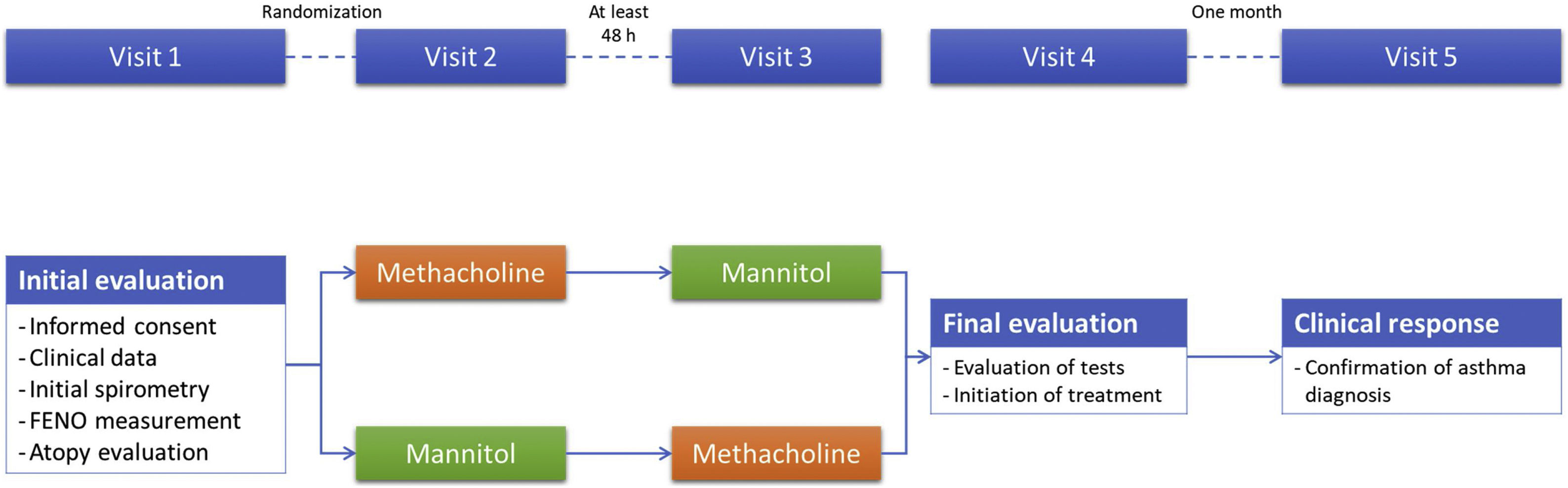
MethodsObservational, cross-over, randomized trial evaluating adult cases with suspected asthma, naïve to treatment, with normal pre-bronchodilator spirometry, and negative bronchodilator test. Patients were randomized to start with methacholine or mannitol. The diagnosis of bronchial asthma was confirmed if there was a good functional and clinical response to one month with twice daily formoterol/budesonide 9/320. The diagnostic profile and the concordance were calculated. Factors associated with a positive provocation test were entered into a multivariate binomial logistic regression analysis, and classification trees were created for both tests.
ResultsThe study included 108 cases (50.0% diagnosed with asthma and 51.9% cases starting with methacholine). The percentage of cases positive to methacholine and mannitol were 30.6% and 25.0% respectively. Kappa values were 0.40 (p<0.001). The diagnostic profile for methacholine was sensitivity 59.3% and specificity 98.1%, while for mannitol it was sensitivity 48.1% and specificity 98.1%. Variables associated with a positive methacholine response included sex, atopy, FEV1, FEV1/FVC and FENO, whereas they were FEV1/FVC and FENO for mannitol. A FENO value>26ppb, FEV1≤103.3% and female sex correctly classified 78.7% of methacholine responders. FENO value>26ppb was enough to correctly classify 81.5% of mannitol responders.
ConclusionsOur study confirms the diagnostic profile of methacholine and mannitol challenge tests and describes the variable associated to their positivity with new proposed cutoff values.
Despite the remarkable advances made in the characterization of bronchial asthma over the last decades, the identification of asthma cases continues to be a challenge for the clinician. Current clinical guidelines recommend a battery of diagnostic tests to try to reveal the reversibility or variability of bronchial obstruction in order to confirm the diagnosis in a suggestive clinical scenario.1 One of the tools available for diagnosing asthma is the nonspecific provocation test. Currently, there are several ways to evaluate this bronchoprovocation, of which the methacholine test and mannitol provocation are the preferred methods due to their easy implementation and accessibility.2
Various studies have comparatively evaluated these two diagnostic techniques, methacholine vs. mannitol, in the diagnosis or evaluation of patients with bronchial asthma and controls.3–8 Of note, a recent metanalysis evaluating 6 studies including 565 cases showed that mannitol presented a better diagnostic performance than methacholine for identifying bronchial hyperresponsiveness in asthma.9 However, most of these studies were carried out in patients already diagnosed with bronchial asthma, in those under treatment with inhaled steroids, in non-randomized design, with different gold standards for asthma diagnosis or with a small number of subjects.
Consequently, despite the available evidence, the question of the role of nonspecific bronchial provocation tests in the initial diagnosis of steroid-naïve subjects with symptoms suggestive of asthma remains largely unexplored.10 By means of a randomized cross-over design, we aimed to evaluate the role of methacholine and mannitol challenge testing in the diagnostic algorithm of subjects with suspected asthma, and explore the factors associated with this broncoprovocation response.
MethodsThis is an observational, cross-over, randomized trial evaluating adult cases sent from Primary Care to our specialized asthma outpatient clinic with suspected asthma (consisting of cough, respiratory wheezing predominantly at night, and dyspnea on exertion), naïve to treatment, with a normal pre-bronchodilator spirometry, and a negative bronchodilator test according to the Spanish National Asthma Guideline (GEMA) criteria. All the patients had been advised to undergo a nonspecific bronchoprovocation test as part of the diagnostic strategy to diagnose asthma according to current recommendations.1,11 Exclusion criteria included any contraindication against provocation tests, the impossibility of performing a proper spirometric maneuver, smokers or ex-smokers with cumulated consumptions>10 pack-years. Recruitment took place between March 2010 and February 2012.
After signing a written informed consent (Fig. 1), the clinical variables were collected, atopy explored with a skin prick test or specific IgE in blood analysis, a pre and post-bronchodilator spirometry was performed, and the patients were randomized to decide which test they would start with, methacholine or mannitol. A time lapse of at least 48h between provocation tests was required. The second provocation tests were conducted without the technician knowing the result of the previous test. The clinical variables collected included age, sex, smoking history, previous atopy, asthma control test,12 Fractional Exhaled Nitric Oxide (FENO) expressed in ppb, body mass index, and pre and post-bronchodilator spirometry according to the current recommendations at the time of the study.13 After performing both diagnostic tests, all patients started with inhaled dry-power treatment with formoterol/budesonide at a dose of 2 inhalations of 320/9 mcg every 12h, with treatment continuing for at least one month. All the patients were trained in the correct use of the inhalers by the nursing staff.
Provocation tests were performed and interpreted according to current regulations.14,15 In the case of the methacholine bronchoconstriction test, we used the technique of methacholine inhaled by dosimeter based on tidal breathing and the result depended on the cumulative dose of methacholine. Methacholine (Provocholine®, Methapharm, Inc. Brantford, ON, Canada) was delivered from a nebulizer (Jaeger APS Pro) by the dosimeter method with doubling dose increments from the initial dose of 0.0312mg/ml. Spirometry was performed within 3min. The response to methacholine was expressed as the dose required to provoke a 20% fall in FEV1 from the pre-challenge value (PD20). A positive test was considered when this drop occurred with a dose of less than 0.4mg of methacholine.
The mannitol test was carried out using an Osmohale kit (MIAS Pharma Limited, Dublin, Ireland). The FEV1 was measured 60s after each mannitol dose (0, 5, 10, 20, 40, 80, 160, 160, 160mg). The subjects were asked to exhale completely before taking a controlled deep inspiration from the device and to hold their breath for 5s then exhale through their mouth before removal of the nose clip. Sixty seconds after inhalation of the 0mg capsule the FEV1 was measured in duplicate. The highest of these values was taken as the baseline FEV1 and was used to calculate the target FEV1 value. The response to mannitol was expressed as the dose required to provoke a 15% drop in FEV1 compared to baseline (PD15) or an incremental decrease in FEV1≥10% between two consecutive doses. The test is considered negative when a cumulative dose of 635mg of mannitol has been administered and the FEV1 has not fallen by ≥15% from baseline.14
The diagnosis of bronchial asthma was confirmed, according to the diagnostic algorithm of the GEMA,11 in those patients in whom a good response to treatment was confirmed after 4 weeks, measured by an increase in forced expiratory volume in 1s (FEV1) compared with the previous one by more than 200ml or an increase in FEV1 of <200ml, and the disappearance of all previous respiratory symptoms.
EthicsThis study followed the recommendations of the Declaration of Helsinki for studies with human beings and was approved by the hospital's ethics and clinical research committee. The patients’ personal data were kept under strict confidentiality in compliance with the provisions of Organic Law 3/2018, dated December 5 and the Regulation (EU) 2016/679 of the European Parliament and Council.
Statistical approachThe sample size was estimated before beginning the study. We decided that at least 97 patients should be included in the study in order to achieve a power of 80% and to detect differences in the contrast of the null hypothesis by means of a McNemar test for two related samples, using a 5% level of significance and a 20% proportion in the reference group to obtain a clinically relevant difference. Therefore, given an estimated 10% loss, the minimum sample size was set at 107 patients.
Statistical analysis was performed with IBM SPSS Statistics (Armonk, New York, USA), version 26.0. The p value was set at 0.05. Data were described using absolute frequencies, with relative frequencies in parentheses or using the mean and standard deviation in parentheses, according to the nature of the variable. For comparisons between two groups (with and without asthma; starting with mannitol or methacholine; positive or negative provocation test results), Chi-square and Student's t tests were used for independent data, according to the nature of the variables. The McNemar test was used to compare paired qualitative data such as the appearance of clinical symptoms during both tests. However, normality was independently checked beforehand for both groups with the Kolmogorov–Smirnoff test and homoscedasticity was evaluated with the Levene test. If comparisons in parametric tests were not suitable, non-parametric inferential studies were used. Specifically, the comparison between the 4 test response groups was performed using the Kruskal–Wallis test.
To analyze the concordance between diagnostic tests, the Kappa index was performed. For diagnostic confirmation, we carried out an analysis of sensitivity, specificity, negative and positive predictive values of methacholine and mannitol, using the asthma diagnosis as the reference test, including 95% confidence intervals (CI). Youden's J statistic was estimated to summarize the performance of a diagnostic test. Since the increased specificity of these test has been previously described,16 we constructed Receiver Operating Characteristics (ROC) curves for both the methacholine and mannitol tests and evaluated the best cutoff point for the drop in FEV1 in both tests in order to maintain a specificity of 90%.
Next, we evaluated which factors were associated with a positive provocation test response. Using bivariate analyses, we explored all the clinical variables collected to see which ones were associated with a positive test, and any significant ones were entered into a multivariate binomial logistic regression analysis with the intro method to estimate odds ratios (OR) and 95%CI. Finally, for those variables which remained significant in the model, we plotted separate classification trees for methacholine and mannitol.
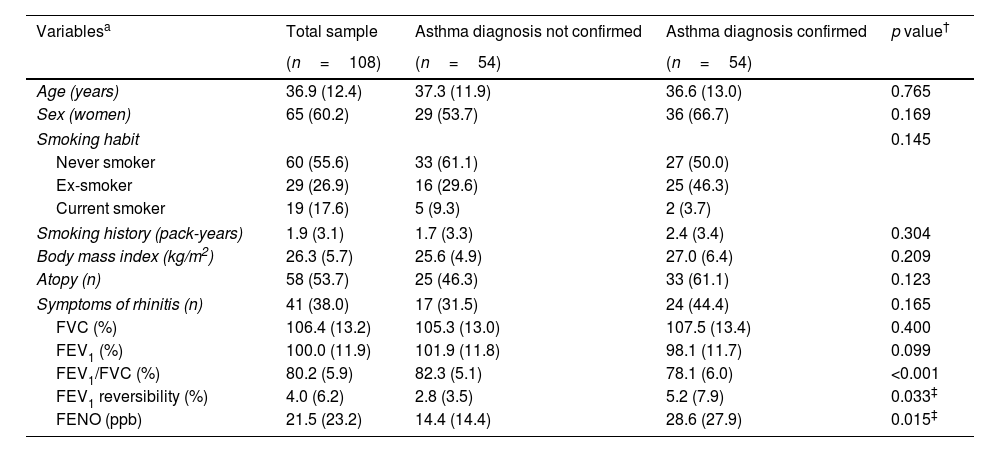
ResultsThe study group consisted of 108 cases, of which 54 (50.0%) were finally diagnosed with asthma. A descriptive summary of the sample can be found in Table 1. This was a young cohort, with low smoking history, and preserved lung function as per inclusion criteria. Although the cases diagnosed with asthma presented significatively different values of FEV1/FVC, FEV1 reversibility and FENO, all these cases were within the normal values.
Description of the patients studied.
| Variablesa | Total sample | Asthma diagnosis not confirmed | Asthma diagnosis confirmed | p value† |
|---|---|---|---|---|
| (n=108) | (n=54) | (n=54) | ||
| Age (years) | 36.9 (12.4) | 37.3 (11.9) | 36.6 (13.0) | 0.765 |
| Sex (women) | 65 (60.2) | 29 (53.7) | 36 (66.7) | 0.169 |
| Smoking habit | 0.145 | |||
| Never smoker | 60 (55.6) | 33 (61.1) | 27 (50.0) | |
| Ex-smoker | 29 (26.9) | 16 (29.6) | 25 (46.3) | |
| Current smoker | 19 (17.6) | 5 (9.3) | 2 (3.7) | |
| Smoking history (pack-years) | 1.9 (3.1) | 1.7 (3.3) | 2.4 (3.4) | 0.304 |
| Body mass index (kg/m2) | 26.3 (5.7) | 25.6 (4.9) | 27.0 (6.4) | 0.209 |
| Atopy (n) | 58 (53.7) | 25 (46.3) | 33 (61.1) | 0.123 |
| Symptoms of rhinitis (n) | 41 (38.0) | 17 (31.5) | 24 (44.4) | 0.165 |
| FVC (%) | 106.4 (13.2) | 105.3 (13.0) | 107.5 (13.4) | 0.400 |
| FEV1 (%) | 100.0 (11.9) | 101.9 (11.8) | 98.1 (11.7) | 0.099 |
| FEV1/FVC (%) | 80.2 (5.9) | 82.3 (5.1) | 78.1 (6.0) | <0.001 |
| FEV1 reversibility (%) | 4.0 (6.2) | 2.8 (3.5) | 5.2 (7.9) | 0.033‡ |
| FENO (ppb) | 21.5 (23.2) | 14.4 (14.4) | 28.6 (27.9) | 0.015‡ |
Values expressed in absolute (relative) frequencies or by mean (standard deviation), depending on the nature of the variable. FVC: forced vital capacity. FEV1: forced expiratory volume in 1s. FENO: Fractional Exhaled Nitric Oxide.
† Calculated with chi-squared or unpaired Student's t test, unless otherwise specified.
‡ Calculated with Mann–Whitney test.
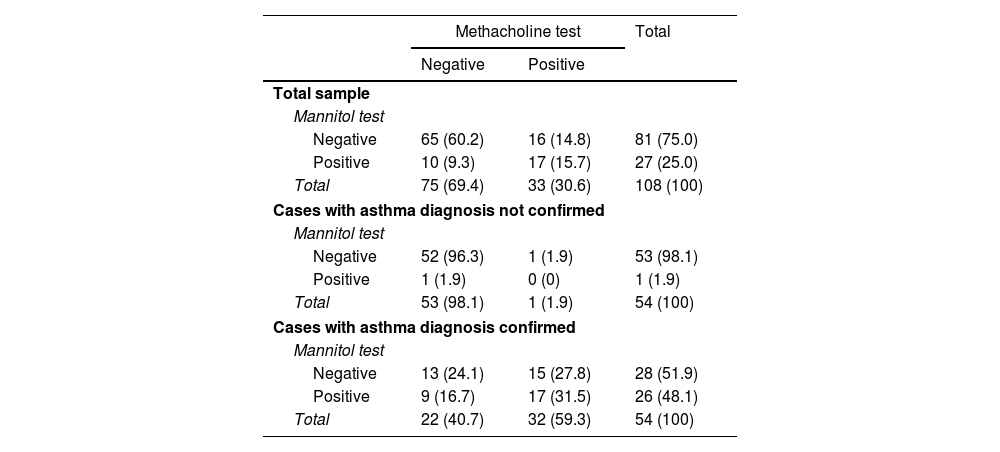
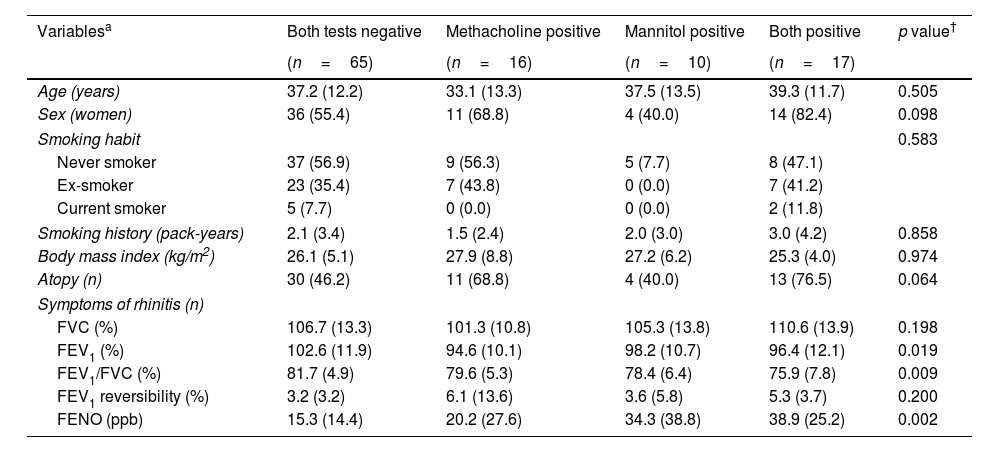
After randomization, 56 (51.9%) cases started with the methacholine test, while 52 (48.1%) started with mannitol. There were no differences in any of the baseline variables (see Table 1) according to these groups (data not shown). The distribution of the results of these tests is summarized in Table 2. From the total sample, 33 (30.6%) cases showed a positive methacholine test, with 27 (25.0%) showing a positive mannitol test. Kappa values were 0.40 (p<0.001) for the whole cohort, −0.2 (p=0.890) for cases without a confirmed asthma diagnosis and 0.1 (p=0.377) for cases with a confirmed asthma diagnosis. The baseline descriptions of the clinical and functional baseline data according to the test response are shown in Table 3.
Results of the non-specific bronchoprovocation tests.
| Methacholine test | Total | ||
|---|---|---|---|
| Negative | Positive | ||
| Total sample | |||
| Mannitol test | |||
| Negative | 65 (60.2) | 16 (14.8) | 81 (75.0) |
| Positive | 10 (9.3) | 17 (15.7) | 27 (25.0) |
| Total | 75 (69.4) | 33 (30.6) | 108 (100) |
| Cases with asthma diagnosis not confirmed | |||
| Mannitol test | |||
| Negative | 52 (96.3) | 1 (1.9) | 53 (98.1) |
| Positive | 1 (1.9) | 0 (0) | 1 (1.9) |
| Total | 53 (98.1) | 1 (1.9) | 54 (100) |
| Cases with asthma diagnosis confirmed | |||
| Mannitol test | |||
| Negative | 13 (24.1) | 15 (27.8) | 28 (51.9) |
| Positive | 9 (16.7) | 17 (31.5) | 26 (48.1) |
| Total | 22 (40.7) | 32 (59.3) | 54 (100) |
Results expressed as absolute counts with percentages in parentheses. Percentages refer to total sample in each group.
Baseline characteristics of the patients according to the test response.
| Variablesa | Both tests negative | Methacholine positive | Mannitol positive | Both positive | p value† |
|---|---|---|---|---|---|
| (n=65) | (n=16) | (n=10) | (n=17) | ||
| Age (years) | 37.2 (12.2) | 33.1 (13.3) | 37.5 (13.5) | 39.3 (11.7) | 0.505 |
| Sex (women) | 36 (55.4) | 11 (68.8) | 4 (40.0) | 14 (82.4) | 0.098 |
| Smoking habit | 0.583 | ||||
| Never smoker | 37 (56.9) | 9 (56.3) | 5 (7.7) | 8 (47.1) | |
| Ex-smoker | 23 (35.4) | 7 (43.8) | 0 (0.0) | 7 (41.2) | |
| Current smoker | 5 (7.7) | 0 (0.0) | 0 (0.0) | 2 (11.8) | |
| Smoking history (pack-years) | 2.1 (3.4) | 1.5 (2.4) | 2.0 (3.0) | 3.0 (4.2) | 0.858 |
| Body mass index (kg/m2) | 26.1 (5.1) | 27.9 (8.8) | 27.2 (6.2) | 25.3 (4.0) | 0.974 |
| Atopy (n) | 30 (46.2) | 11 (68.8) | 4 (40.0) | 13 (76.5) | 0.064 |
| Symptoms of rhinitis (n) | |||||
| FVC (%) | 106.7 (13.3) | 101.3 (10.8) | 105.3 (13.8) | 110.6 (13.9) | 0.198 |
| FEV1 (%) | 102.6 (11.9) | 94.6 (10.1) | 98.2 (10.7) | 96.4 (12.1) | 0.019 |
| FEV1/FVC (%) | 81.7 (4.9) | 79.6 (5.3) | 78.4 (6.4) | 75.9 (7.8) | 0.009 |
| FEV1 reversibility (%) | 3.2 (3.2) | 6.1 (13.6) | 3.6 (5.8) | 5.3 (3.7) | 0.200 |
| FENO (ppb) | 15.3 (14.4) | 20.2 (27.6) | 34.3 (38.8) | 38.9 (25.2) | 0.002 |
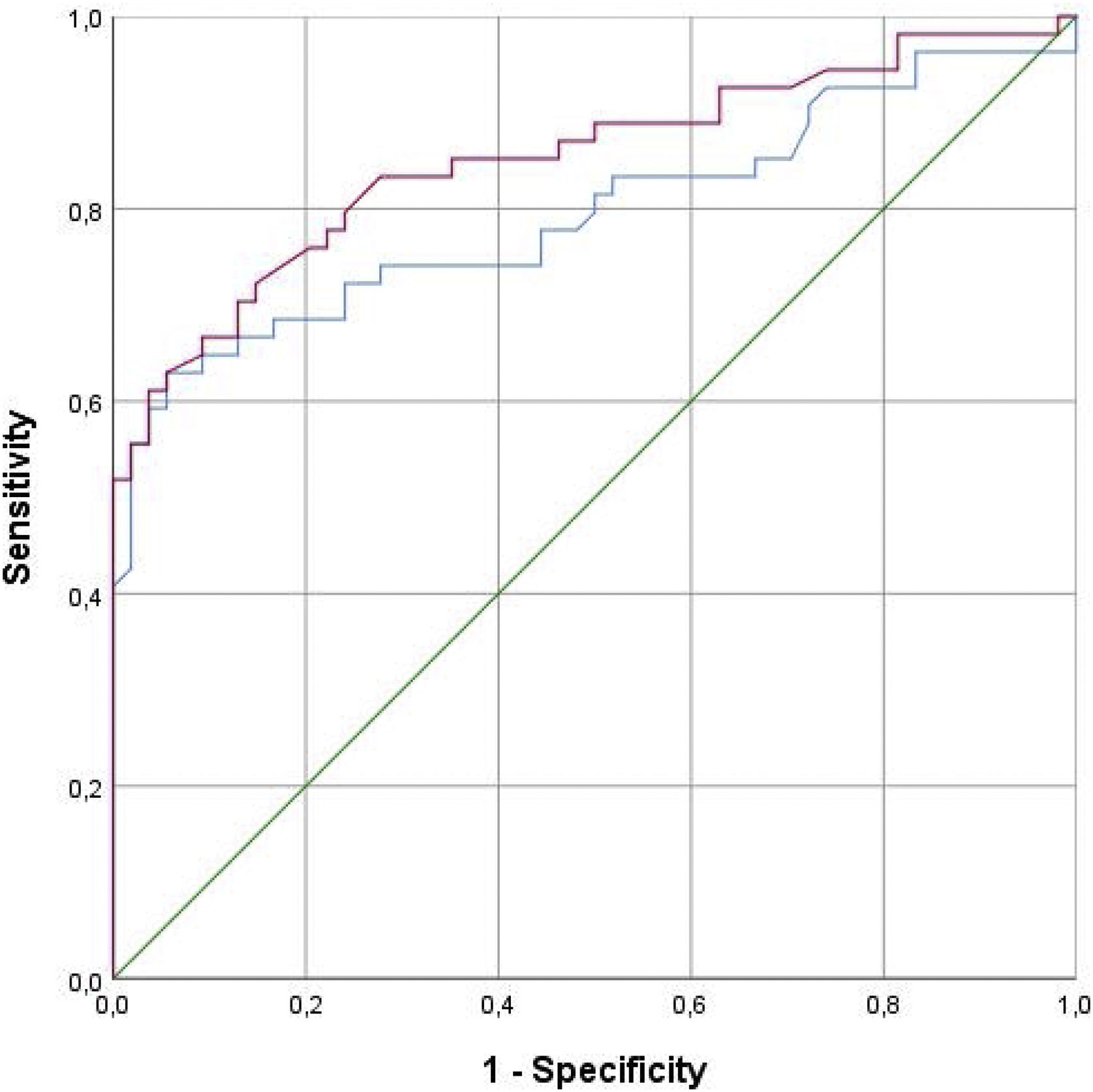
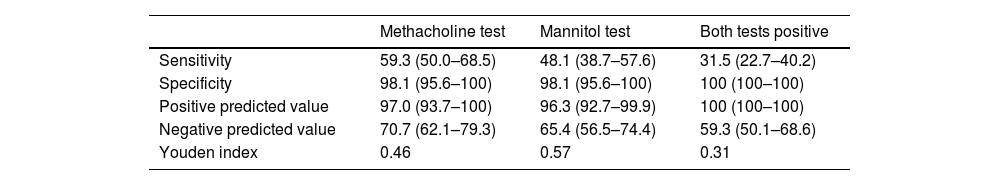
The diagnostic profile of the two tests is summarized in Table 4. Both tests showed a high specificity with higher negative predictive values. The ROC curve is shown in Fig. 2. The methacholine test produced a slightly greater area under the curve of 0.852 (p<0.001; 95%CI 0.777–0.956) than the mannitol test 0.795 (p<0.001; 95%CI 0.706–0.883). The best cutoff point for the mannitol test was a drop in FEV1 of 10%, which showed a sensitivity of 64.8% and a specificity of 90.7%, while the best cutoff point for the methacholine test was an FEV1 drop of 11.4%, which yielded a sensitivity of 66.07% and a specificity of 90.7%. The diagnostic performance of methacholine for a 10% drop in FEV1 during the methacholine test was 72.2% sensitivity and 85.2% specificity.
Diagnostic profile of non-specific bronchoprovocation tests.
| Methacholine test | Mannitol test | Both tests positive | |
|---|---|---|---|
| Sensitivity | 59.3 (50.0–68.5) | 48.1 (38.7–57.6) | 31.5 (22.7–40.2) |
| Specificity | 98.1 (95.6–100) | 98.1 (95.6–100) | 100 (100–100) |
| Positive predicted value | 97.0 (93.7–100) | 96.3 (92.7–99.9) | 100 (100–100) |
| Negative predicted value | 70.7 (62.1–79.3) | 65.4 (56.5–74.4) | 59.3 (50.1–68.6) |
| Youden index | 0.46 | 0.57 | 0.31 |
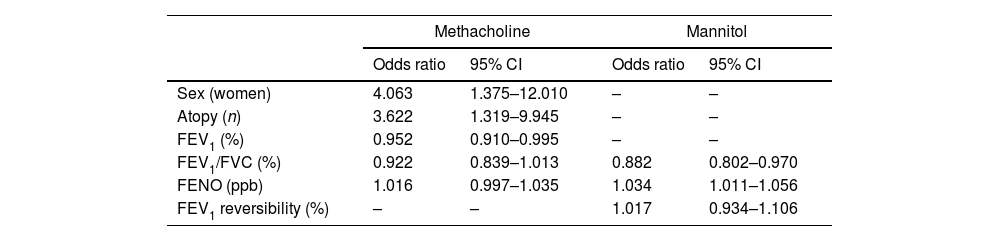
The characteristics of responders to both tests are summarized in the online supplement: Table 1S for the methacholine test and Table 2S for the mannitol test. The variables associated with a positive response included sex, atopy FEV1 (%), FEV1/FVC (%) and FENO for methacholine but only FEV1/FVC (%) and FENO for mannitol. The result of the multivariate analysis is shown in Table 5, which summarizes the different contributions of the variables in the model.
Adjusted odds ratios for positivity in provocation tests.
| Methacholine | Mannitol | |||
|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Sex (women) | 4.063 | 1.375–12.010 | – | – |
| Atopy (n) | 3.622 | 1.319–9.945 | – | – |
| FEV1 (%) | 0.952 | 0.910–0.995 | – | – |
| FEV1/FVC (%) | 0.922 | 0.839–1.013 | 0.882 | 0.802–0.970 |
| FENO (ppb) | 1.016 | 0.997–1.035 | 1.034 | 1.011–1.056 |
| FEV1 reversibility (%) | – | – | 1.017 | 0.934–1.106 |
CI: confidence intervals. FENO: Fractional Exhaled Nitric Oxide. FEV1: forced expiratory volume in 1s. FVC: forced vital capacity.
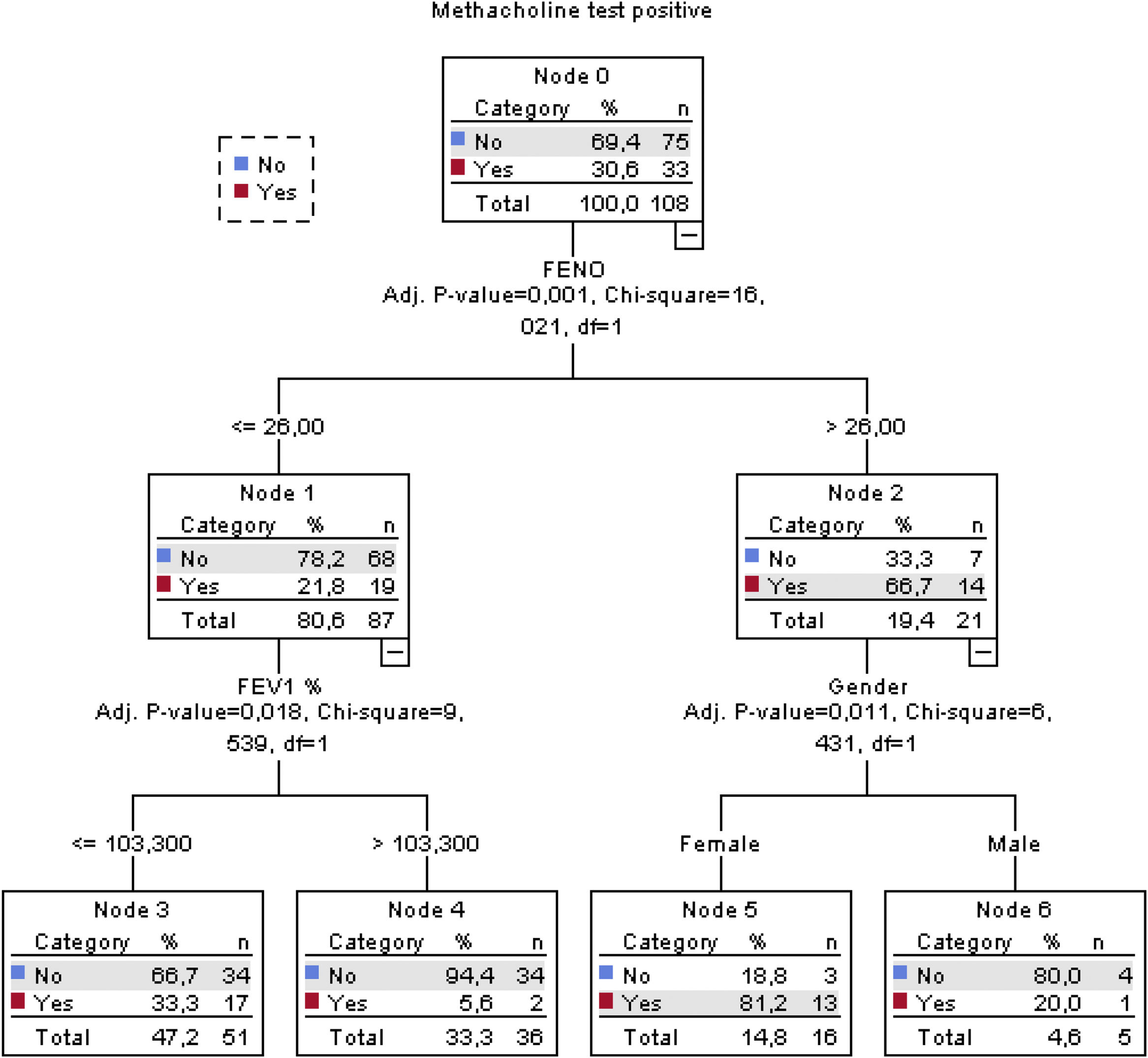
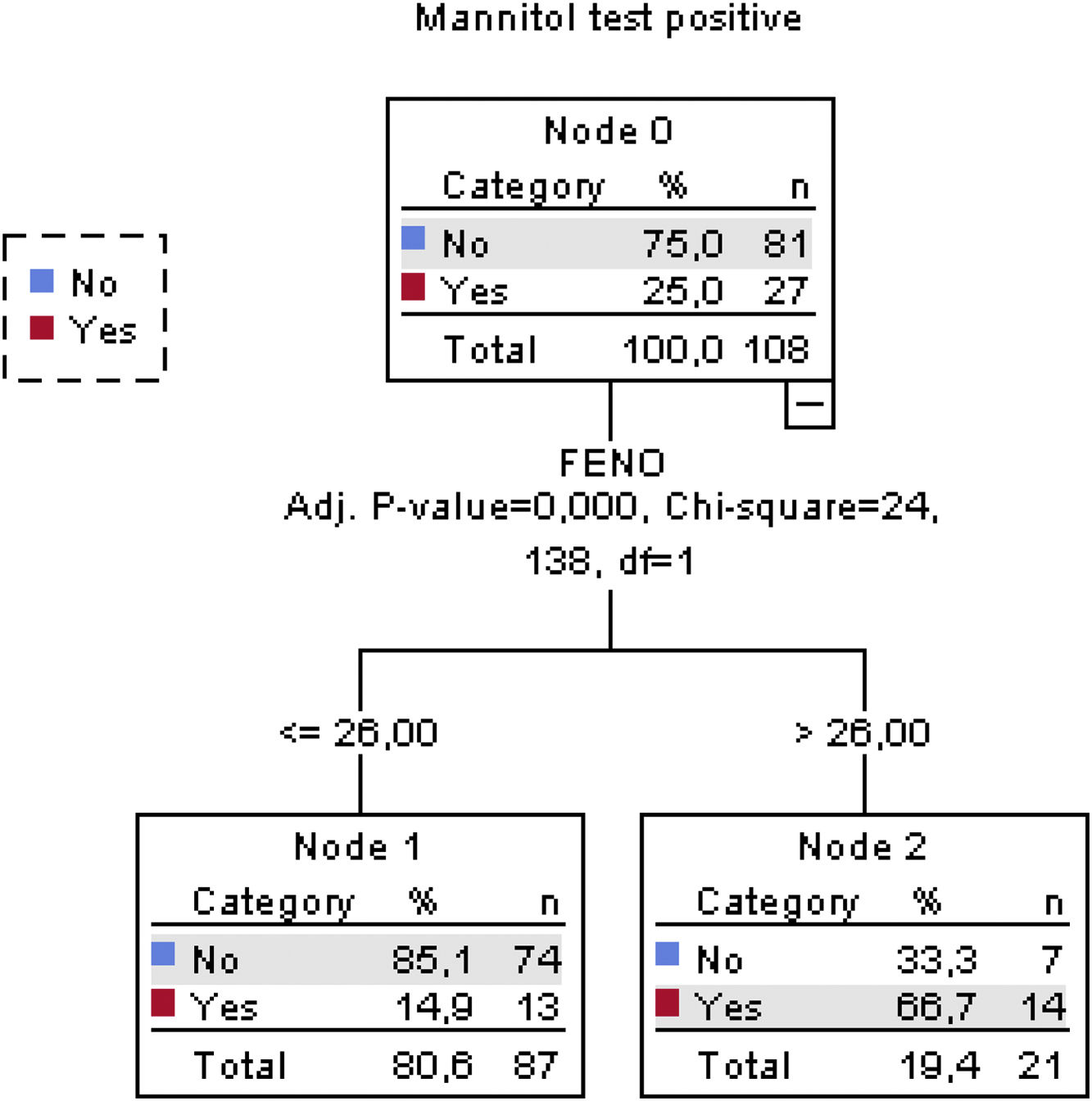
The classification tree for the methacholine test is shown in Fig. 3. Altogether, a FENO value>26ppb, FEV1≤103.3% and female sex correctly classified methacholine responders with an overall percentage of 78.7%. The classification tree for the mannitol test is shown in Fig. 4. Here, only a FENO value>26ppb was enough to correctly classify mannitol responders with an overall percentage of 81.5%.
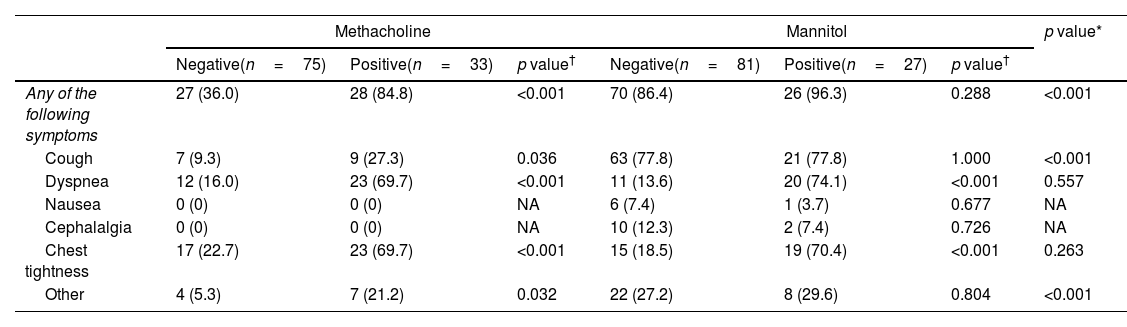
The presentation of clinical symptoms during the test is shown in Table 6. Altogether, 55 (50.9%) of the methacholine test and 96 (88.9%) of the mannitol tests presented clinical symptoms during the test. The mannitol test presented cough and dyspnea more frequently than the methacholine test. Also, cases with a positive test presented symptoms more frequently in the methacholine challenge test than with mannitol.
Clinical symptoms during both provocation tests.
| Methacholine | Mannitol | p value* | |||||
|---|---|---|---|---|---|---|---|
| Negative(n=75) | Positive(n=33) | p value† | Negative(n=81) | Positive(n=27) | p value† | ||
| Any of the following symptoms | 27 (36.0) | 28 (84.8) | <0.001 | 70 (86.4) | 26 (96.3) | 0.288 | <0.001 |
| Cough | 7 (9.3) | 9 (27.3) | 0.036 | 63 (77.8) | 21 (77.8) | 1.000 | <0.001 |
| Dyspnea | 12 (16.0) | 23 (69.7) | <0.001 | 11 (13.6) | 20 (74.1) | <0.001 | 0.557 |
| Nausea | 0 (0) | 0 (0) | NA | 6 (7.4) | 1 (3.7) | 0.677 | NA |
| Cephalalgia | 0 (0) | 0 (0) | NA | 10 (12.3) | 2 (7.4) | 0.726 | NA |
| Chest tightness | 17 (22.7) | 23 (69.7) | <0.001 | 15 (18.5) | 19 (70.4) | <0.001 | 0.263 |
| Other | 4 (5.3) | 7 (21.2) | 0.032 | 22 (27.2) | 8 (29.6) | 0.804 | <0.001 |
Values expressed as absolute count (relative frequencies). Percentages refer to total number of the patients in each column.
In the present study, we have explored the different behavior of two non-specific provocation tests in the initial diagnosis of adult asthma. Our data indicate that both tests have a similar diagnostic profile, with a slightly better profile for the methacholine test, and that a number of variables (FENO value>26ppb, FEV1≤103.3% and female sex) are associated with the response to the provocation tests, which may help us to prioritize these diagnostic tests in a diagnostic algorithm for asthma. Finally, clinical symptoms during the tests showed a different profile.
Despite its complexity compared with other simpler tests, the evaluation of the airway hyperresponsiveness continues to be one of the best ways of establishing a diagnosis of asthma.1,11,17 Notably, at present there is no gold standard universally accepted for asthma diagnosis. Here, we used the one proposed by the Spanish GEMA document,11 which states that a diagnosis will be confirmed when a good response to treatment is evident. Unfortunately, GEMA does not indicate how or when to assess this response to treatment, leaving the question unresolved. To ensure that we selected responders, we used a demanding combination of functional and clinical improvement. Additionally, we took a limit of 4 weeks following GEMA recommendations. However, the response to inhaled steroids in asthma is heterogenic in specific forms of asthma. This may lead to an underestimation of the true asthma population in our cohort. Other studies have identified other gold standards. Anderson et al.8 evaluated the diagnostic profile of both methacholine and mannitol using exercise-induced bronchoconstriction or a clinical diagnosis of asthma as the reference. Interestingly, the diagnostic profile of mannitol and methacholine presented a worse specificity if exercise-induced bronchoconstriction is used as a reference.
For the analysis of bronchial hyperresponsiveness, direct agents can be used on the smooth muscle cells of the airway, particularly with inhaled methacholine or histamine.19–21 On the other hand, indirect tests can be performed with different stimuli.19,22,23 These indirect tests act by increasing the release of mediators in the airway. Both methods cause bronchoconstriction, but their different mechanism of action has led to compare their role in the diagnosis of bronchial asthma. A key feature of indirect tests is that response is based on the presence of active airway inflammation that is sensitive to inhaled corticosteroids. However, this is not essential for direct tests where inflammation sensitive to inhaled corticosteroids may or may not be present and remodeling or reduced airway caliber are important determining factors. Therefore, since their mechanism of action and their diagnostic profiles are different, it could be considered to use complementarily different bronchoprovocation tests within the study of bronchial asthma.
Although methacholine and mannitol have been compared in different studies and clinical situations, the evaluation of these tests in the initial diagnosis of asthma has been addressed in three studies. The first was by Cancelliere et al.10 These authors evaluated 28 consecutive patients with persistent asthma-like symptoms. They found that bronchial challenge with mannitol yielded similar results to methacholine, in the initial diagnosis of asthma. However, methacholine was better tolerated and had fewer side effects than mannitol. Interestingly, they were unable to calculate the diagnostic profile since the same provocation test was used as the gold standard for diagnosing asthma. The second study was by Kim et al.6 These authors evaluated a total of 50 asthmatic adults and 54 controls. However, the diagnosis of asthma was established before the study to include those patients who had been diagnosed with asthma by specialist physicians and had used asthma medication for 6 months before the enrollment. Therefore, the design was not exactly the same as ours. In this study the authors found a sensitivity and specificity for the mannitol challenge of 48.0% and 92.6%, respectively, with the figures for methacholine 42.0% and 98.1%, respectively. Finally, Porpodis et al.7 evaluated 88 subjects who presented with asthma related symptoms and were not on any anti-asthma medication. In this study, the asthma diagnosis was established by the combination of at least a +12% (and at least a 200ml) increase in baseline FEV1 after albuterol, along with new symptoms of coughing, wheezing, or shortness of breath over the past month, and no previous diagnosis of asthma. They found that methacholine presented a sensitivity of 62.6% and specificity of 85.7%, whereas mannitol presented a sensitivity of 64.1% and specificity of 95.2%.
The identification of variables associated with positive methacholine and mannitol tests may help prioritize tests. The role of FENO in identifying the response to provocation tests, as well as the cut-off point of 26ppb consistent for the two tests, is striking considering that the first studies demonstrating that FENO predicted a clinical response to inhaled steroids set their cutoff value at 47ppb.24 The role of FENO in the diagnosis of asthma is also open to certain debate.25 FENO assesses a molecule released from the epithelium due to a TH2 inflammatory profile that has a wide range of responses in subjects with active asthma who are naïve to inhaled steroids. Therefore, despite the fact that FENO has been modestly associated with sputum and blood eosinophils in asthma,26 the GINA guidelines do not recommend the use of FENO as a confirmatory test for asthma diagnosis due to its unspecificity and interaction with external factors such as tobacco and viral infections.1,27,28 Despite these arguments, our data show that there is a relationship between FENO and the positive response to provocation tests. In this regard, recently, blood eosinophils, FENO and small airways dysfunction markers have been linked to the level of response to methacholine in patients with asthma-like symptoms and no airflow limitation in spirometry.29 The cutoff value of 26ppb we have identified is also an interesting issue open to debate.31 However, previous FENO cut-off points are not always supported by sufficient evidence. More information therefore needs to be found about the diagnostic profile of FENO based on data.
The clinical response presented a different profile between the two tests. In a challenge test, it is to be expected that patients will experience respiratory symptoms as part of the positive response to the test, as already described.7,8 Interestingly with the mannitol test presented more frequent symptoms, although these were not always related to a positive result in the test. While cough is clearly more prevalent in the mannitol test but with no difference in the test result, dyspnea and chest tightness are more frequent with a positive test unrelated to the test evaluated. These results are similar to the study of Anderson et al. who found a frequency of 93% with cough and 43% with occasional cough which, likewise, did not interfere with the test result.8 The cough response to indirect provocation tests appears to be of clinical significance, as recently summarized with cough-provocation tests with hypertonic aerosols.30
The present study has certain strengths, including the sample size calculated by an ad hoc estimation and the randomization of the procedures. However, there are a number of methodological considerations that must be taken into account to interpret our results properly. First, this was a study performed in adults, excluding children. Second, this cohort explores the left part of the diagnostic algorithm of GEMA in which the performance of a non-specific bronchial provocation test is indicated. Consequently, we are evaluating a specific type of patient in a precise clinical scenario. Third, how the methacholine test is performed is a key issue. It has been shown that the method of methacholine inhalation (total lung capacity or tidal breathing) affects the results of the test.18 Here, we used the methacholine inhaled by dosimeter technique with tidal breathing. When methacholine is inhaled by deep total lung capacity inhalations, deep inhalation inhibition of bronchoconstriction leads to a marked loss of diagnostic sensitivity when compared to tidal breathing inhalation methods.
In conclusion, our study confirms the diagnostic profile of methacholine and mannitol challenge tests and describes the variable associated to their positivity with new proposed cutoff values. The low degree of agreement between the two tests suggests that, in the case of suspected asthma with one of these two tests resulting negative, the physician should consider performing the other test too, due to the discrepancy between the results. Future studies should prospectively evaluate the effectiveness, cost and safety of each diagnostic test as a way of organizing asthma diagnosis better in specific clinical scenarios, evaluate the role as a diagnostic tool for asthma identifying evidence-based cutoff points and advance in a personalized diagnostic approach that would allow us to create patient-tailored cost-effective diagnostic algorithms.
FundingThis project has been funded by a research grant from the Neumosur Foundation (project no. 01.2009).
Conflicts of interestMARF JFMG has received honoraria as a speaker, scientific advisor or participant in clinical studies in the last 3 years sponsored by Chiesi, GSK, Astra Zeneca, Novartis and ALK.
JFMG has received honoraria as a speaker, scientific advisor or participant in clinical studies in the last 3 years sponsored by AstraZeneca, Bial, Chiesi, Gebro Pharma, GlaxoSmithKline, Novartis, Sanofi and Teva.
JLLC has received honoraria during the last 3 years for lecturing, scientific advice, participation in clinical studies or writing for publications for (alphabetical order): AstraZeneca, Bial, Boehringer, Chiesi, CSL Behring, Faes, Ferrer, Gebro, Grifols, GSK, Megalabs, Menarini, and Novartis.
FJAG has participated in speaking activities and advisory boards, and provided consultancy services sponsored by AstraZeneca, ALK, Bial, Boehringer-Ingelheim, Chiesi, GSK, Mundipharma, Novartis, Orion-Pharma and Sanofi during the period 2017–2022.
The rest of the authors declare no conflicts of interest.