The emergence of immunotherapy for the treatment of autoimmune diseases has revolutionized the management of many diseases in recent years, including multiple sclerosis (MS). Ponesimod is a highly selective sphingosine-1-phosphate receptor (S1PR) modulator that shows high-affinity binding to subtype 1 (S1PR1) in lymphocytes and other cells. Ponesimod prevents lymphocytes from leaving the lymph nodes, thus reducing their concentration in peripheral blood. Its mechanism of action in MS has not been fully clarified, but its therapeutic effects are probably associated with the reduced migration of lymphocytes to the central nervous system (CNS).1,2 This drug has been marketed in Spain for the treatment of recurrent forms of MS since 2022.
We report a little-known adverse effect in a young woman with no respiratory history who, after starting ponesimod, developed symptoms of progressive dyspnea reaching modified scale of the Medical Research Council (mMRC) grade 3 and mild obstruction with bronchial reversibility in spirometry.
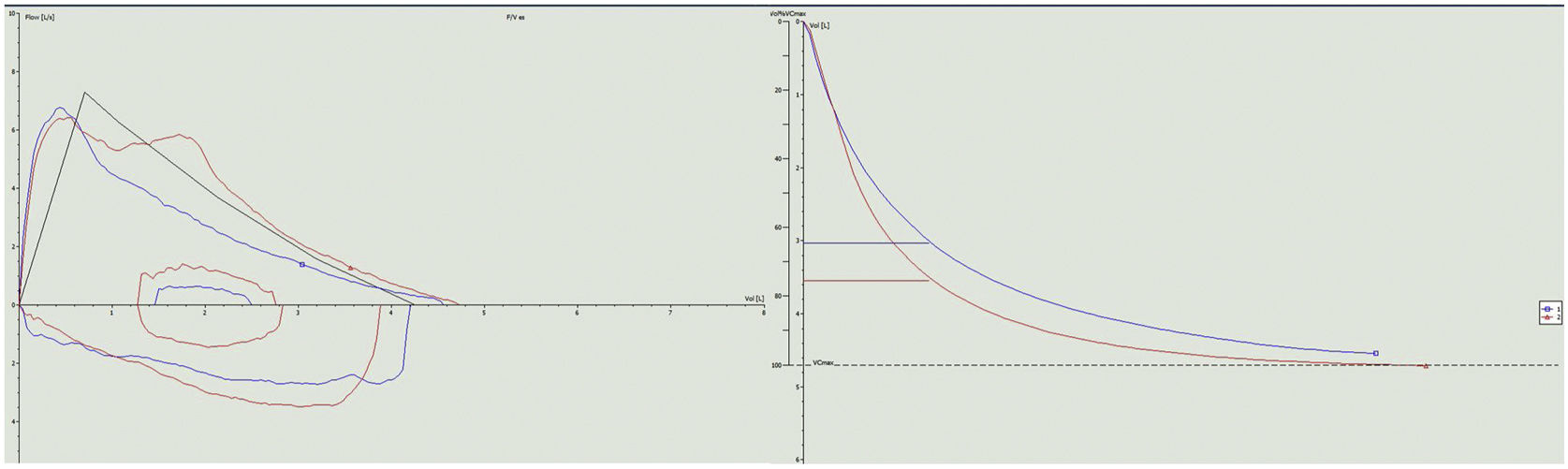
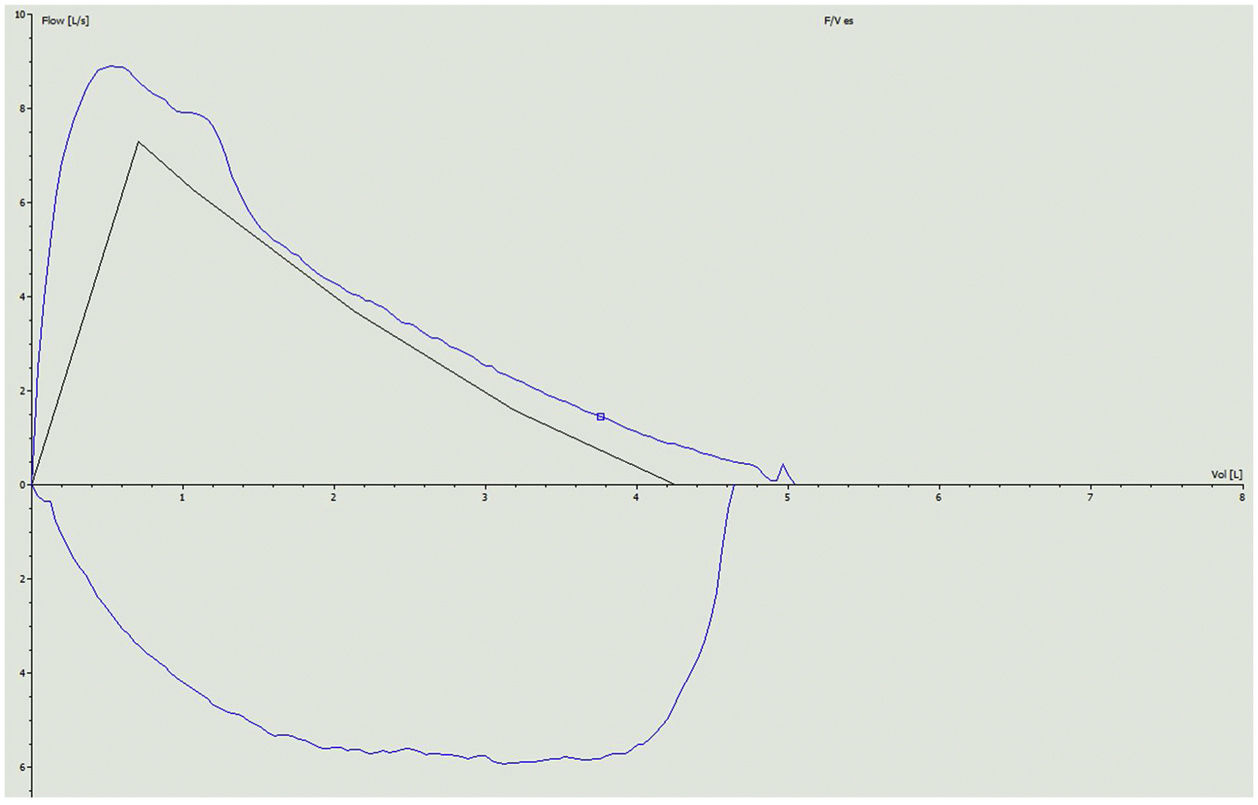
Our patient was a 34-year-old woman diagnosed in 2011 with relapsing-remitting MS. After therapeutic failure with cladribine, she began treatment with ponesimod in January 2023. Neurological examination on starting ponesimod was normal. Within 3 weeks of administration, the patient developed dyspnea that limited her activities of daily living, eventually reaching mMRC grade 3. In view of these symptoms, she was referred to the respiratory medicine department for further investigation. The patient reported no cough, expectoration, wheezing, chest tightness or other symptoms associated with her dyspnea. She had no history of asthma or bronchial hyperreactivity during respiratory infections. She had no known respiratory history, no history of nasal polyposis, no NSAID intolerance, no IgE elevation or peripheral eosinophilia, and had never required bronchodilator treatment. Spirometry was performed, showing FVC 4550ml (107%), FEV1 3040ml (86%), FEV1/FVC 0.67 (80% predicted), consistent with mild obstructive ventilatory airflow changes. A bronchodilator challenge with 400mcg of salbutamol produced a significant 16.7% improvement in FEV1 from baseline (510ml), 14.5% improvement in predicted value, and reversibility of obstruction with normalization of FEV1/FVC (0.75) (Fig. 1). A pulmonary carbon monoxide diffusion (DLCO) test was performed with a result of 107% (within normal limits) and an exhaled nitric oxide (FeNO) measurement of 23ppb. The study was completed with high-resolution computed tomography of the chest that revealed no significant radiological alterations.
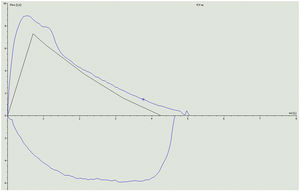
Given these findings, we initially planned to start bronchodilator treatment, but the temporal relationship of symptoms with the start of ponesimod suggested that bronchoconstriction was due to this therapy, so we decided to discontinue the drug and replace it with ofatumumab, an anti-CD20 monoclonal antibody, without adding bronchodilator therapy. The patient's respiratory symptoms resolved 2 weeks after discontinuing ponesimod. Spirometry was repeated showing parameters within normal limits: FEV1 5060ml (118%), FEV1 3760ml (106%), FEV1/FVC 74% (89% predicted) (Fig. 2).
The patient's good progress following discontinuation of ponesimod, with resolution of dyspnea and normalization of spirometry values, suggests that her symptoms and respiratory function changes were directly related to the drug.
Sphingosine-1-phosphate (S1P) is a key mediator that exerts a multitude of effects on different organs through the different S1P receptor subtypes that are expressed in a wide variety of tissues. These regulate a large range of biological functions, including lymphocyte trafficking, vascular permeability, and bronchial tone. In experimental trials in mice, S1P has been seen to intervene in multiple stages of the asthmatic response through activation of the S1PR1 and S1PR3 receptor subtypes. In this way, it promotes the contraction of smooth muscle cells in the respiratory tract and regulates the activation of mast cells and eosinophils, producing an inflammatory response and remodeling of bronchial tissue.3
According to the package insert, ponesimod should be used with caution in patients with severe respiratory disease, pulmonary fibrosis and chronic obstructive pulmonary disease, because dose-dependent reductions in FEV1 and reductions in lung carbon monoxide diffusion capacity (DLCO) have been observed, mainly during the first month after starting treatment.4
No cases have been published to date on symptomatic bronchoconstriction due to ponesimod in patients with multiple sclerosis in routine clinical practice, but 2 cases of worsening asthma caused by fingolimod have been reported.5,6 This is a drug with a mechanism of action similar to ponesimod that involves sphingosine-1-phosphate receptor modulation.
This case report highlights the risk of bronchoconstriction associated with ponesimod, which can produce symptoms that can limit normal activity, even in patients without a history of respiratory disease. Therefore, all patients treated with ponesimod should be closely monitored for the appearance of respiratory symptoms and spirometry should be considered to determine respiratory function during treatment, not only in patients with a history of previous respiratory disease, but also in individuals who report dyspnea or other respiratory symptoms. It is important to be aware of this possible adverse effect when prescribing ponesimod to patients with multiple sclerosis.
FundingThis study has not received funding of any kind.
Conflict of InterestsThere are no conflicts of interest directly or indirectly related to the contents of the manuscript.