The expression of cyclooxygenase 2 (COX-2) is usually increased in inflammation and cancer. This study examines the expression of COX-2 in the lung of chronic obstructive pulmonary disease (COPD) patients with lung cancer.
MethodsWe studied 44 male patients with bronchial cancer (27 squamous carcinoma and 17 adenocarcinoma). Samples were obtained from the pulmonary parenchyma, from the bronchial mucosa adjacent to the tumor and from the tumor itself. Lung tissue specimens from 14 patients with pneumothorax were used as control. The mRNA and the COX-1 and COX-2 proteins were assessed by RT-PCR and Western blot, respectively.
ResultsCOX-1 and COX-2 mRNA levels were significantly higher in the lung parenchyma of COPD patients than in the control subjects. COX-2 mRNA levels were also higher in the lung parenchyma than in both tumor and airway tissue samples procured from COPD patients. There were no differences in the COX-2 mRNA levels between squamous carcinoma and adenocarcinoma. In contrast, COX-2 protein levels were significantly higher in tumors than in lung parenchyma and airways. COX-2 protein levels were higher in adenocarcinoma compared with squamous carcinoma.
ConclusionThis study shows that in COPD, the pathway of cyclooxygenase is activated and associated with an increase in the expression of COX-2 in lung tumors. These observations suggest that COX-2 is possibly involved in the association between COPD and cancer.
La ciclooxigenasa 2 (COX-2) está aumentada en la inflamación y en el cáncer. En este estudio se evaluó la expresión de la COX-2 en el pulmón y en el cáncer bronquial de pacientes con EPOC.
MétodosSe estudiaron 44 pacientes varones con cáncer bronquial (27 escamosos y 17 adenocarcinomas). Se obtuvieron muestras del parénquima pulmonar, de la mucosa bronquial adyacente al tumor y del tumor mismo. El tejido pulmonar de 14 pacientes con neumotórax se empleó como control. El ARNm y la proteína de la COX-1 y de la COX-2 se midieron mediante RT-PCR y western blot, respectivamente.
ResultadosLos niveles de ARNm de la COX-1 y de la COX-2 en el parénquima de los pacientes con EPOC fueron superiores a los de los controles. Los niveles del ARNm de la COX-2 en pacientes con EPOC fueron más altos en el parénquima pulmonar que en las vías aéreas y los tumores. No hubo diferencias en los niveles del ARNm de la COX-2 entre escamosos y adenocarcinomas. En contraste, la proteína de COX-2 mostró niveles más altos en los tumores que en el parénquima y las vías aéreas. Los niveles de la proteína de COX-2 fueron más altos en los adenocarcinomas que en los carcinomas escamosos.
ConclusiónEste estudio muestra que en la EPOC la vía de la ciclooxigenasa está activada y asociada a un aumento en la expresión de la COX-2 en los tumores. Cabe la posibilidad de que la COX-2 esté involucrada en la asociación de EPOC y cáncer.
Abundant scientific information upholds the existence of a relationship between inflammation and the initiation and progression of various types of cancer, among these bronchial cancer.1 It is also known that chronic obstructive pulmonary disease (COPD) and bronchial cancer are closely related with smoking. Tobacco use could be the link that would explain this association, as it is considered as the common denominator responsible for both processes. Several studies, however, have shown that the severity of the bronchial obstruction, evaluated by measuring forced expiratory volume in one second (FEV1), is an independent factor for the development of bronchial cancer in smokers.2–4 This observation suggests that there is a connection between the two diseases, which could be independent from the action of tobacco. Given that the onset of cancer is associated with chronic inflammation and COPD is an inflammatory disease of the airways, it is possible that the inflammatory process present in smokers that develop bronchial obstruction is the link between COPD and cancer. Several studies point to lipid inflammatory mediators among the possible factors responsible for the relationship between inflammation and cancer.
Lipid mediators are formed in inflammatory processes from arachidonic acid (AA). AA is released from the deposits of phospholipids by the action of phospholipase A2, which can be activated by different stimuli, among which are cytokines, microorganisms, and different types of cell lesions. AA can be metabolized by three main pathways: that of cyclooxygenase (COX), that of lipoxygenase and that of the chromosome P450, by means of which a family of products called eicosanoids is formed. The family of the eicosanoids includes prostaglandins (PG) made by the action of the COX. There are two types of COX that participate in the synthesis of PG: COX-1 and COX-2. COX-1 is normally present in the cells and is in charge of physiological functions; in contrast, COX-2 is usually undetectable in normal situations, but it is rapidly induced in the cells when they are subject to inflammatory stimuli.5
The prostaglandins generated by the two COX enzymes are PGD2, PGE2, and PGF2α. Each one participates in the regulation of the inflammatory responses.5
The expression of COX-2 is higher in several human cancers, including bronchial cancer. The increased expression of COX-2 leads to an increase in the production of PGE2, a fact that has been demonstrated in colorectal, pancreatic, and lung cancers.6 PGE2 stimulates angiogenesis, cell invasion, the formation of metastasis and cell survival, the latter inhibiting apoptosis.6,7
Previous studies have demonstrated in COPD patients an increase in the production of PGE2 associated with the up-regulation of COX-2.8,9 Another interesting observation is that smoking also increases the expression of COX-2 and the production of PGE2.10,11
Taking all these data into account and associating them with the known interdependence, which is among smoking, COPD, and bronchial cancer, it is possible that the induction of COX-2, together with the increased production of PGE2, could be the common denominator for all of them.
In this study, we establish the hypothesis that, in smokers who are present with bronchial inflammation associated with COPD, the increased production of PGE2 makes them prone to developing bronchial cancer. Given that the increase in PGE2 is associated with higher COX-2 expression, we decided to study the expression of this enzyme in the tumor tissue, the bronchial mucosa adjacent to the tumor and in the pulmonary parenchyma distal to the area of the tumor invasion.
The specific objectives of the study are: (a) to evaluate and compare the expression of COX-2 in the lungs of COPD patients and subjects not affected by COPD; (b) to evaluate and compare the expression of COX-2 in the lung parenchyma, the airways, and the lung tissue of patients with COPD and bronchial cancer; (c) to analyze and compare the expression of COX-2 depending on the histologic type of the bronchial cancer, as well as its relationship with the degree of extension of the process.
MethodsPatientsA total of 44 male patients, smokers or ex-smokers with an age of 65±6 (mean±standard deviation), who underwent lung resection pulmonary for bronchial cancer, were included in the study. The histological classification of the tumors and the degree of extension was carried out in accordance with the guidelines established to this end.12,13 Twenty-seven tumors were classified as squamous and 17 as adenocarcinoma. The extension study of the tumors classified these into: stage IB, 29 patients; stage IIA, 5 patients; stage IIB, 1 patient; stage IIIA, 6 patients; and stage IIIB, 4 patients. All the patients presented a history of chronic bronchitis, evolving over the course of several years. The study of the lung function showed that the majority had bronchial obstruction. The FEV1 of the group of the 44 patients was 60%±12% predicted value. Forty-two of the 44 patients met the criteria that classified them as having COPD. The two patients who were not diagnosed with COPD were not excluded from the study given their history of chronic bronchopathies.14
For the control population, we used a group of 14 male non-smoker patients with a mean age of 34±6 who underwent surgical interventions in order to treat recurrent spontaneous pneumothorax. The age of the control group was significantly lower (P<.05) than that of the group of COPD patients with bronchial cancer. None of the control subjects were studied with spirometry; therefore, although it is very improbable, we cannot rule out that they may have had a previous, undetected obstructive disease.
The study was approved by the Ethics Committee of our institution and all the patients signed an informed consent accepting the use of samples obtained for the aims of the study.
Tissue Sample ProcessingWe obtained samples from the tumor, from the area adjacent to the cancer-free bronchus and from the pulmonary parenchyma that was as distal as possible from the tumor. The tumor samples were obtained from areas that were not necrotic in appearance. The tissue samples from the control subjects that were affected by pneumothorax were obtained from an area close to the resected lung in the intervention. The samples were immediately frozen in liquid nitrogen and stored at −80°C until their processing and analysis.
Isolation of RNA, Reverse Transcription (RT) and Polymerase Chain Reaction (PCR)The frozen tissue samples were homogenized and the total ribonucleic acid (RNA) was isolated by means of a commercial method of extraction (TRI-reagent, MRC, Cincinnati, Ohio). The integrity of the RNA and the reverse transcription of the total RNA to DNA were done according to a method previously described in detail.14 The expression of messenger RNA (mRNA) of COX-1 and COX-2 was analyzed with semi-quantitative RT-PCR with primers and previously published reaction conditions.15
Western BlottingFifty-five micrograms of protein from each sample were analyzed in polyacrylamide sulfate electrophoresis gels using the Novex X-cell II® System and a 7% Tris–acetate separation gel. The proteins, once separated, were transferred to nitrocellulose membranes. The staining of the membranes was done with Ponceau S (Sigma Chemical, St. Louis, MO) with the aim of evaluating the equivalence in the load of each sample and in the transference of the gel. After blocking with 5% powdered milk dissolved in 0.05% PBS containing 1% Tween 20, the membranes were incubated with goat anti-human Cox-1 or Cox-2 polyclonal antibodies (Santa Cruz). The membranes were incubated with a secondary goat antibody for COX-1 and COX-2 conjugated with peroxidase (Santa Cruz). Later, the membranes were processed using chemiluminescent detection reagents (Pierce SuperSignal, Pierce Technology, Rockford, IL) following the instructions of the supplier and using a chemiluminescent film (Hyperfilm NAECL, Amersham). The intensity of the signal of each band in the fluorogram was quantified by means of a densitometry scanning system (BIO1-D program). The evaluation and the comparison of the quantity of proteins were done after normalization, with the densitometry values obtained with the scanning of COX-1 and COX-2 standards that were loaded at the same time as the proteins of the samples to be studied. The results obtained are expressed as percentage of change in optical density (OD).
Statistical AnalysisThe mean values of mRNA and COX-1 and COX-2 proteins of the three groups were compared using a Kruskal–Wallis test. In the case of statistically significant differences, the difference between groups was analyzed with the Mann–Whitney U-test. The correlation between the age of the patients and FEV1 with the levels of expression of COX-1 and COX-2 mRNA and protein were analyzed using Spearman's test. As well, the relationship between the degree of extension of the disease and the COX-1 and COX-2 protein and mRNA values was analyzed using Pearson's test. The data are shown as mean±standard deviation from the mean. A P value<.05 was accepted as the limit for statistical significance.
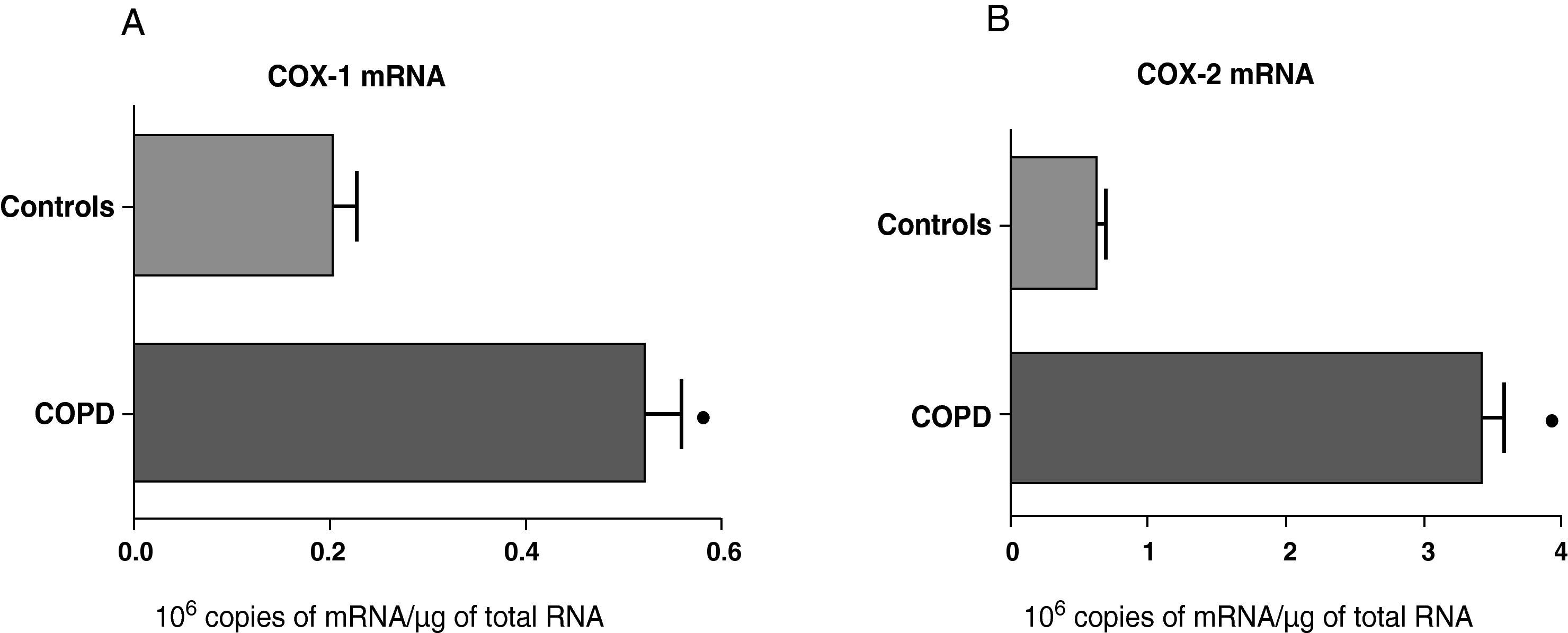
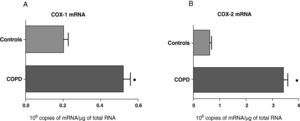
ResultsExpression of COX-1 and COX-2 in Samples of the Pulmonary Parenchyma of Control Subjects and Patients With Bronchial CancerThe levels of expression of COX-1 and COX-2 mRNA in the parenchyma of the patients with bronchial cancer were significantly higher than those found in the control subjects (Fig. 1). However, no differences were observed in the expression of the COX-1 and COX-2 proteins between control subjects and cancer patients (data not shown). As the control group was younger than the study group, we analyzed whether there was a correlation between the enzyme levels and patient age. No correlation was found between age and levels of expression of the two COX (COX-1, r=−0.12; COX-2, r=0.23).
COX-1 (A) and COX-2 (B) mRNA expression in the lung parenchyma of control subjects (n=14) and COPD patients with bronchial cancer (n=44). The means are represented with the standard deviations from the mean. The expression of mRNA is shown as the number of molecules compared with total RNA. ¿ P<.01.
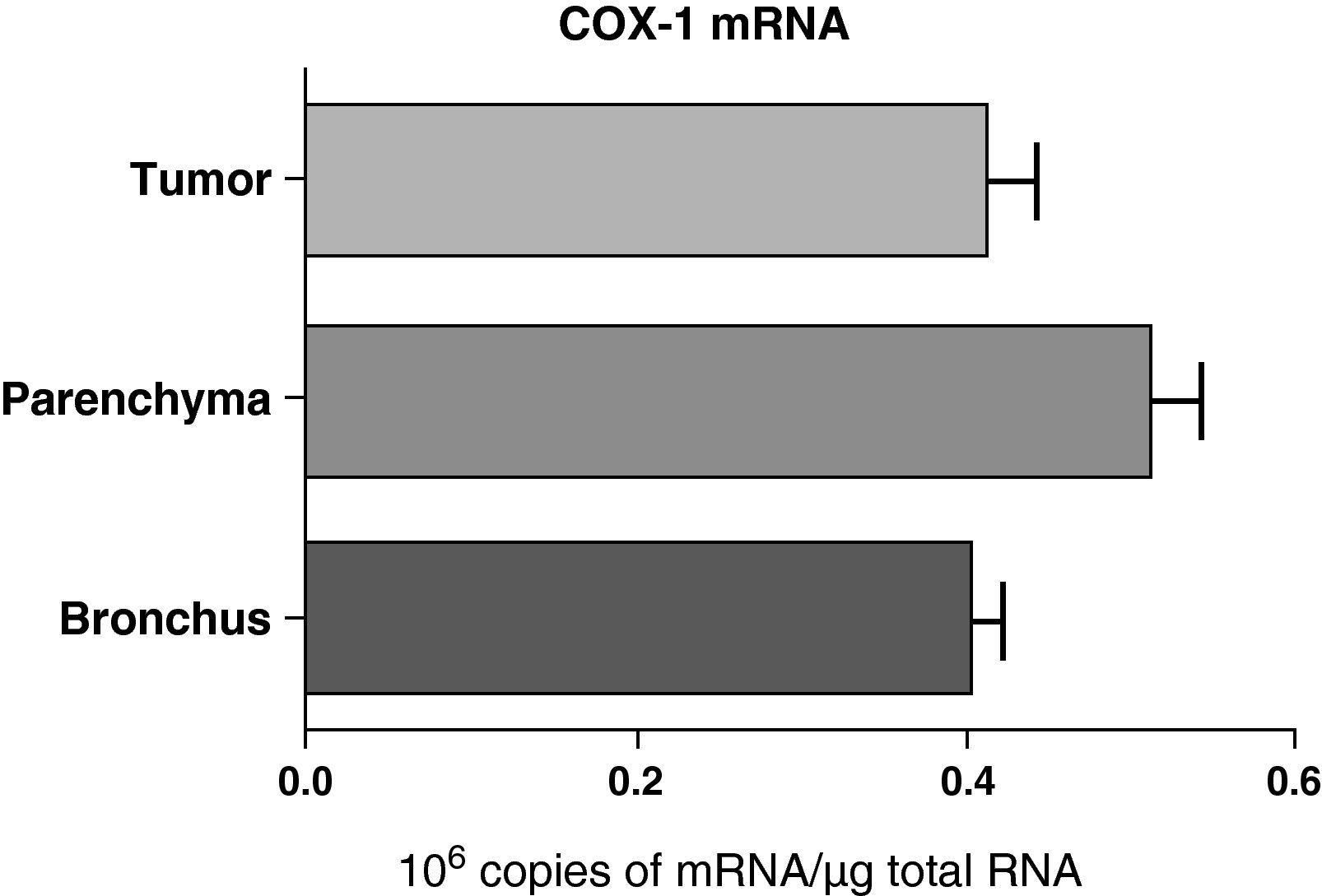
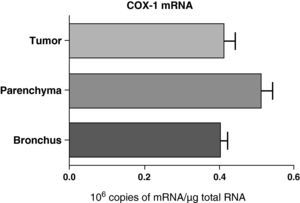
No differences were found in the expression of COX-1 mRNA between the samples obtained from different lung parts: parenchyma, airways, and tumor (Fig. 2). Nor were any differences observed when the samples were compared while taking into account the histologic type of the tumor: squamous or adenocarcinoma (data not shown).
COX-1 mRNA expression in the tumor, lung parenchyma and bronchi of COPD patients with bronchial cancer (n=44). The means are represented with the standard deviations from the mean. The expression of mRNA is shown as the number of molecules compared with total RNA. There were no statistically significant differences.
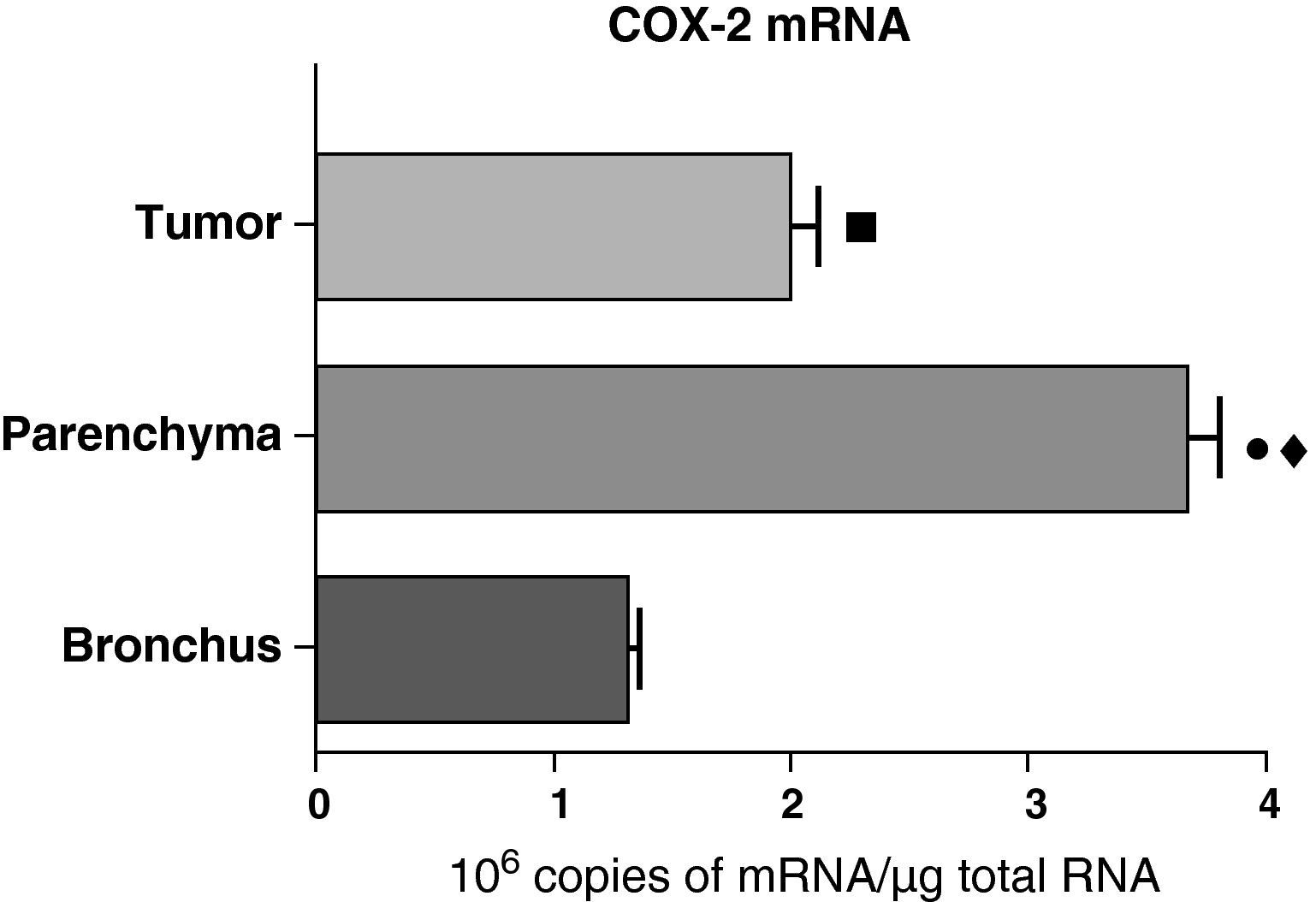
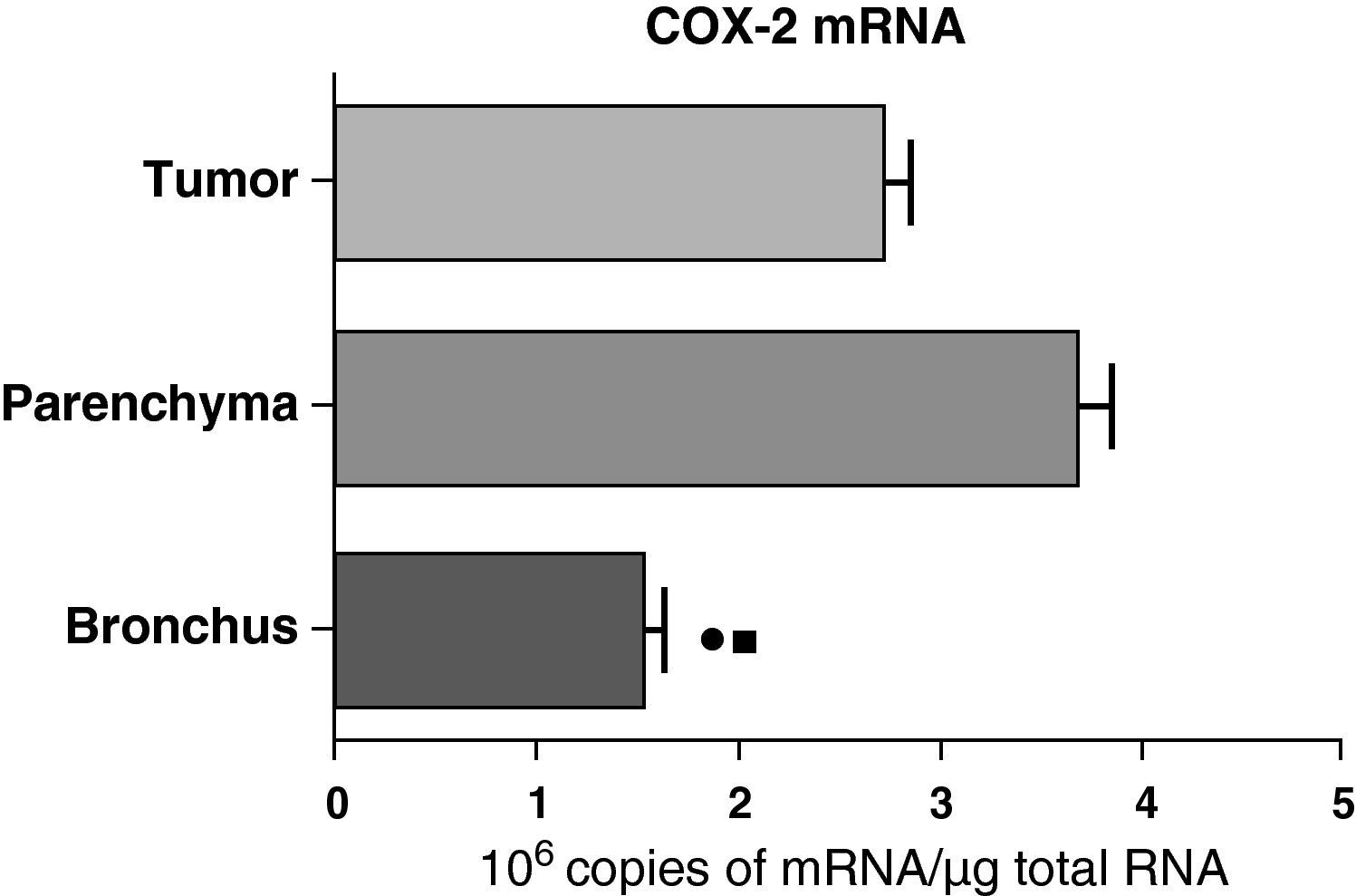
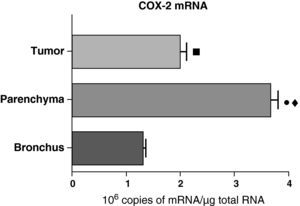
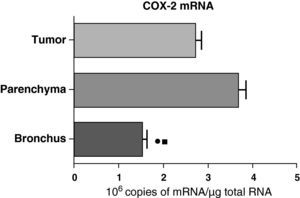
In contrast with the COX-1 mRNA, the expression levels of the COX-2 mRNA in COPD patients were statistically and significantly higher in the pulmonary parenchyma compared with those obtained from the airways and from the tumor samples, both in the lungs of patients with squamous carcinoma (Fig. 3) as well as in patients with adenocarcinoma (Fig. 4). The lower value of COX-2 mRNA was found in the airways in both cases (Figs. 3 and 4). There were no differences in the levels of expression of COX-2 mRNA between squamous and adenocarcinoma cancers.
COX-2 mRNA expression in the tumor, lung parenchyma and bronchi of the COPD patients with squamous carcinoma (n=27). The means are represented with the standard deviations from the mean. The expression of mRNA is shown as the number of molecules compared with total RNA. • P<.001 between parenchyma and tumor; ♦ P<.0001 between parenchyma and bronchus; ¿ P<.01 between tumor and bronchus.
COX-2 mRNA expression in the tumor, lung parenchyma and bronchi of COPD patients with adenocarcinoma (n=17). The means are represented with the standard deviations from the mean. The expression of mRNA is shown as the number of molecules compared with total RNA. • P<.03 between bronchus and tumor; ¿ P<.03 between bronchus and parenchyma. There were no statistically significant differences between parenchyma and tumor.
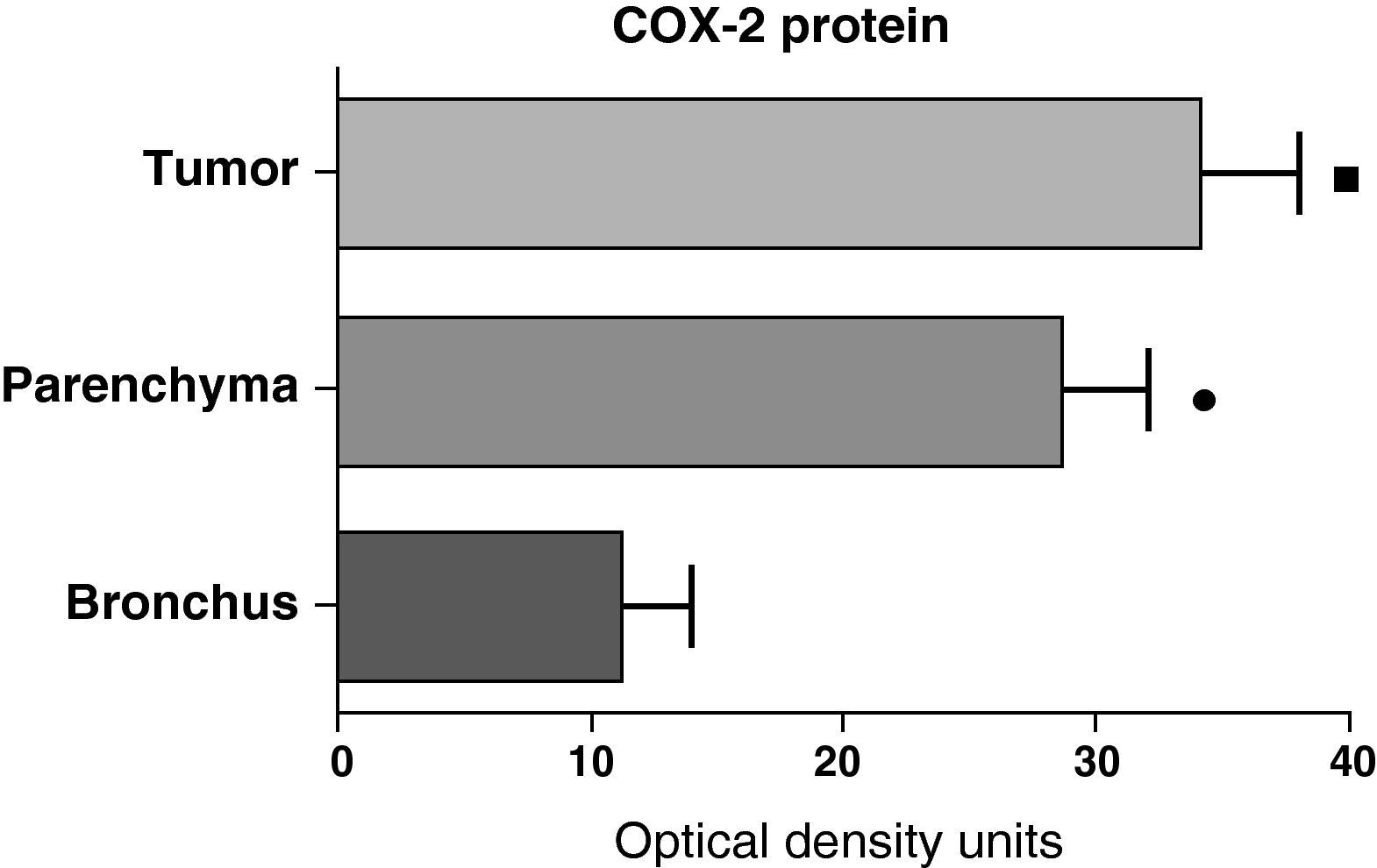
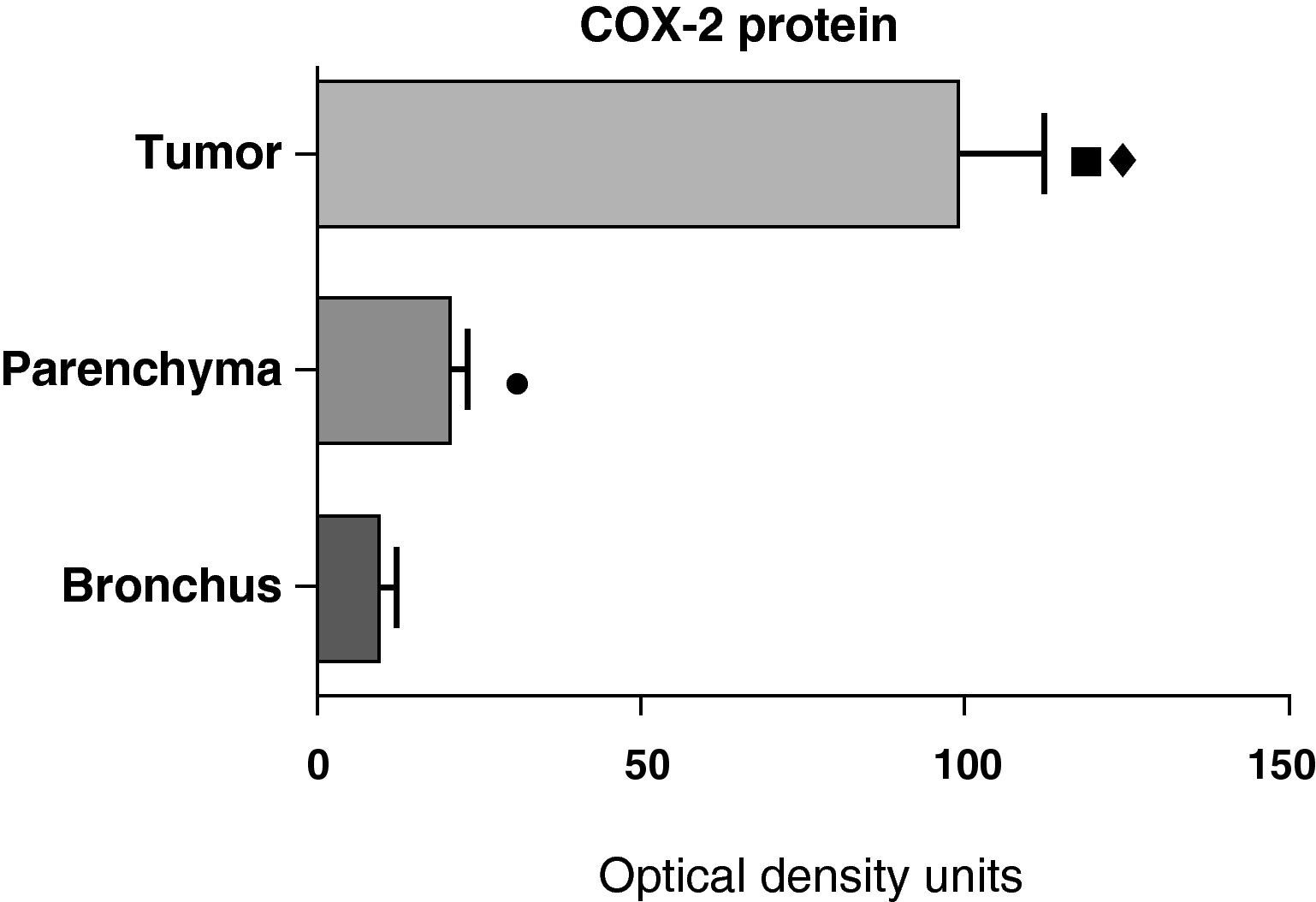
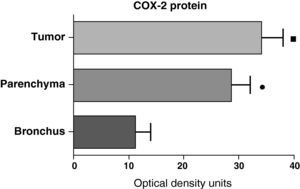
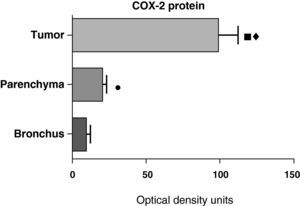
In contrast with the mRNA data, the analysis of the COX-2 protein by Western blot showed significantly higher levels in the tumor samples compared with those detected in the parenchyma and airways. This result was observed in the squamous tumor samples (Fig. 5) as well as in those of patients with adenocarcinoma (Fig. 6)
COX-2 protein expression in the tumor, lung parenchyma and bronchi of the COPD patients with squamous carcinoma (n=27). The means are represented with the standard deviations from the mean. The protein is expressed as optical density units. ¿ P<.01 between tumor and bronchus; • P<.01 between parenchyma and bronchus. There were no statistically significant differences between tumor and parenchyma.
COX-2 protein expression in the tumor, lung parenchyma and bronchi of the COPD patients with adenocarcinoma (n=17). The means are represented with the standard deviations from the mean. The protein is expressed in optical density units. ¿ P<.001 between tumor and bronchus; P<.01 between tumor and parenchyma; ¿ P<.05 between parenchyma and bronchus.
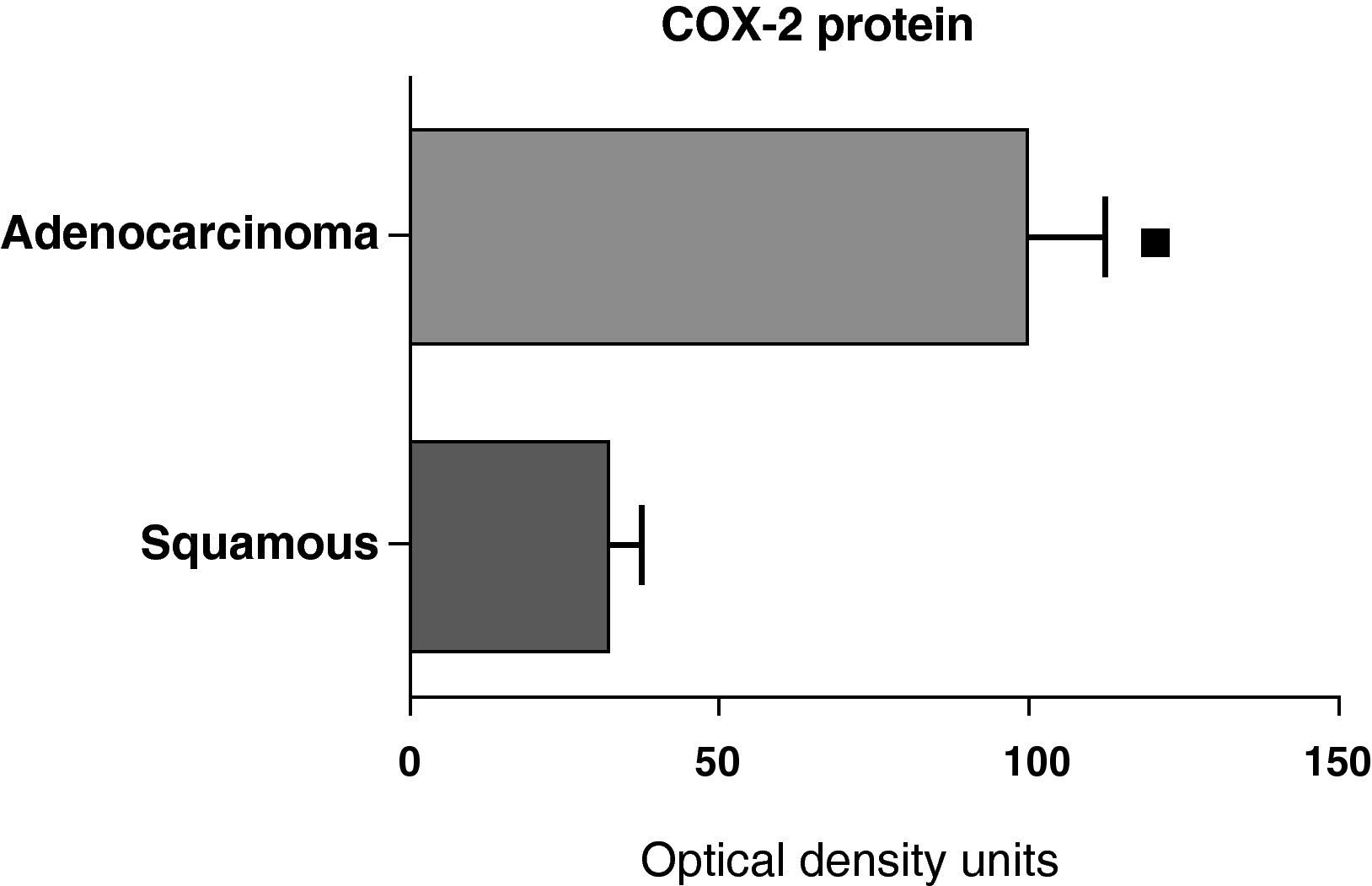
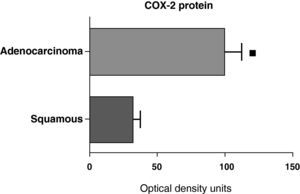
When we compared the expression levels of the COX-2 protein in the parenchyma, airways, and tumor tissue of patients with different histologic types (squamous carcinoma or adenocarcinoma), it was observed that the protein levels were significantly higher in the tumor tissue of the adenocarcinoma than in the squamous cancer (Fig. 7). Nevertheless, there were no differences in protein expression between the samples of the airways or the parenchyma coming from the patients with the two types of tumors (data not shown).
COX-2 protein expression in adenocarcinoma (n=17) and squamous carcinoma.27 The means are represented with the standard deviations from the mean. The protein is expressed in optical density units. ¿ P<.01.
No statistically significant correlations were found between the expression levels of COX-1 and COX-2 mRNA or proteins, and neither with FEV1 nor with the results of the tumor staging (data not shown).
DiscussionGiven that COX-2 seems to be involved in the regulation of various cell processes that are important in the development of cancer, such as angiogenesis, tumor progression, metastases and resistance to anti-neoplastic chemotherapy,16,17 the objective of this study was to research the expression of COX-2 in the lungs of COPD patients affected by bronchial cancer with the aim of analyzing the relationship between the expression of COX-2 in the lung tissue unaffected by the cancer and the neoplastic tissue.
The most relevant findings of our study have been: (a) the expression of COX-1 and COX-2 is up-regulated in the parenchyma of COPD patients; (b) COX-2 mRNA expression is higher in the parenchyma than in the tumor tissue, both from adenocarcinomas as well as squamous carcinomas; (c) The expression of the COX-2 protein is higher in tumor tissue, particularly in the adenocarcinoma samples.
This study confirms that COPD is a disease characterized by the presence of an increase in the activity of the COX pathway of AA metabolism. The higher expression of COX-2 in COPD was described for the first time by our group8 and later confirmed by other researchers.9 These studies demonstrated for the first time that, like COX-2, COX-1 also has a higher expression in COPD. This observation should not be considered exceptional because, although COX-1 is an enzyme that is usually involved in physiological functions, the truth is that in inflammatory processes small changes can be produced in their expression.10,11,18,19 Therefore, it is possible that the increase in the lung production of PGE2 in patients with COPD is in part due to the increase in the metabolic activity of COX-1.
Unlike what happened with mRNA, it was not possible to demonstrate differences in the expression of the COX-1 protein between the pulmonary parenchyma of the control subjects and of the COPD patients. This fact is not exceptional and, when it happens, it is usually due to the differences in the precision and sensitivity of the methods used to measure mRNA and the protein synthesized by the cell ribosomes. PCR is a technique that can detect small changes in mRNA synthesis, while Western blot, which is the technique used to evaluate the quantity of synthesized protein, presents a much lower precision and sensitivity for evaluating slight differences. Other possible reasons, although unlikely, such as the presence of alterations in protein synthesis or alterations in the half life of the proteins generated, could explain the difference in the results obtained with the COX-1 mRNA and protein.
In comparing the expression levels of COX-2 in the tumor tissues, two observations stand out: (a) the protein levels were markedly higher, while the mRNA levels were not as high; (b) The protein levels were much higher in the tissue from adenocarcinoma than from the tissue of squamous carcinoma. These observations suggest that, quite probably, the increased transcription of the COX-2 gene (mRNA) has a less relevant role than the processes of translation (protein synthesis) and post-translation (regulation of the protein half-life) in the increased expression of the COX-2 protein in bronchial tumors.
It is widely accepted that COX-2 plays a relevant role in inflammatory processes, and it is also assumed that the increased expression of this enzyme is mainly responsible for the increased production of PGE2 detected in COPD patients.9 Given the ability of PGE2 to promote oncogenesis, one may be led to think that the increased production of PGE2 as a consequence of the inflammatory response could contribute to the development of cancer in COPD patients.20 One possible sequence in the events that leads to the development of cancer in smokers could be the following: smoking starts up an inflammatory process in the lungs, and the tobacco and the inflammation cause increased COX-2 expression that entails increased production of PGE2, which, with its known capability for inducing oncogenesis and in association with other pro-neoplastic factors, finally promotes the development of cancer situated on an inflamed lung.
If this hypothesis were true, any therapy capable of reducing the synthesis of PGE2 should have a preventive effect on the development of bronchial cancer. Recent studies have shown that low doses of acetylsalicylic acid prevent the development of various cancers, among these bronchial cancer.21,22 Given that one of the actions of acetylsalicylic acid is to inhibit both COX enzymes, thus drastically reducing the synthesis of PGE2, these observations reinforce the hypothesis that indicates the COX pathway as the connection between the inflammation present in COPD and the predisposition of these patients to develop bronchial neoplasms.
One curious fact of this study is the varying degree of COX-2 expression depending on the histologic type. The greater abundance of COX-2 in adenocarcinoma compared with squamous carcinoma has been reported in previous studies.16,23 Given the capacity of low doses of acetylsalicylic acid for preventing the development of cancer, the fact that there are large differences in the levels of COX-2 between squamous and adenocarcinoma emphasize the need to carry out studies in order to know whether the antineoplastic effects of acetylsalicylic are related with the dose of the drug, the histologic type of the tumor or its relationship with the degree of expression of COX-2. The majority of the studies have been done in patients who were receiving treatment with low doses of acetylsalicylic acid with the aim of preventing cardiovascular diseases.21 There exists the possibility that higher doses than those indicated would have greater capacity for cancer prevention, particularly in adenocarcinoma, in which, as demonstrated by this and by other studies, the expression of the COX-2 protein is very high.
It is worth mentioning that the degree of COX expression can have a prognostic value. Previous studies have demonstrated that the up-regulation in the expression of COX-2 is associated with a poorer evolution of bronchial cancer.19,24,25 On the other hand, the inhibition of COX-2 can have not only preventive but also therapeutic effects, a fact supported by the results of studies, which have observed that the inhibition of COX-2 with acetylsalicylic acid or the selective inhibitors of COX-2 (coxibs) improve the therapeutic results when associated with antineoplastic therapies.26,27
In this study, we evaluate the degree COX-2 expression in the bronchial mucosa near the tumor. Our hypothesis established that there should be a relationship between the inflammation present in the airways—with the resulting increase in PGE2 production due to an increase in COX-2 expression—and the later development of cancer on this inflamed area. In accordance with this hypothesis, the bronchial mucosa should be the area of the lungs with a greater expression of COX-2. Surprisingly, the greater expression of COX-2 is found in the parenchyma. The reasons that can explain this finding are unknown for now. Nevertheless, it should be pointed out that the degree of COX-2 expression could not be compared in the bronchial mucosa of the COPD patients with cancer and the control subjects as, although samples were available from the parenchyma of subjects without COPD (affected by pneumothorax), for obvious reasons it was not able to obtain samples of the bronchial tissue of these same individuals. However, previous studies have demonstrated that the COX-2 expression levels are directly related with the degree of malignancy of the bronchial histologic lesions.28
In short, this study demonstrates that COPD is characterized by showing an increase in the activity of the cyclooxygenase pathway, a fact that is associated with an increase in the expression of COX-2 in the tumor tissue, especially in the tissue of adenocarcinoma. As a whole, these data reinforce the idea that inflammation and cancer can be related, and that in this relationship the lipid mediators generated by the COX pathway can play a relevant role. The importance that the inhibition of COX-2 may have in preventing and treating bronchial cancer in COPD patients deserve to be evaluated in prospective studies designed specifically in order to learn their true impact.
Conflict of InterestAuthors have no conflict of interests to declare.
This study was completed with the aid of a grant from the Spanish Fight Against Cancer Association (Asociación Española Lucha contra el Cáncer).
Please cite this article as: Roca-Ferrer J, et al. La ciclooxigenasa-2 está regulada al alza en el pulmón y en los tumores bronquiales de pacientes con enfermedad pulmonar obstructiva crónica. Arch Bronconeumol. 2011. doi:10.1016/j.arbres.2011.05.015.