CURRENT SITUATION AND LONG-TERM CONSEQUENCES OF COVID-19 INFECTION
More infoThe evidence indicates that a short course of systemic corticosteroid in patients with moderate to severe COVID-19 not only reduces escalation of care and improves clinical outcomes1 but also slightly reduces all-cause mortality.2 However, it has also been reported that it might suppress the immune cells and cause a delay of SARS-CoV-2 virus clearance, which may prolong its shedding.3 Since there is still no definitive evidence on whether corticosteroid treatment can cause delayed viral clearance, we conducted a retrospective study to clarify the implication of such treatment on delayed conversion of SARS-CoV-2 RNA nasopharyngeal swabs.
We analyzed data extracted from electronic medical records of 380 consecutive patients admitted to the Cotugno Hospital in Naples, Italy, for suspected or confirmed SARS-CoV-2 infection from 1 March 2020 to 31 May 2020. At the time of hospital admission, each patient was assessed for his/her level of disease severity using the WHO COVID severity categorization,4 and had to have at least one positive real time polymerase chain reaction (RT–PCR) test for SARS-CoV-2, signs of pneumonia on chest CT scan when hospitalized, and also an onset of symptoms no more than 3 days before hospital admission to be included in the analysis. Patients who died before the negative conversion of the viral swab test and those without laboratory tests performed within 48h of admission to hospital were excluded from the analysis.
Taking into account the inclusion and exclusion criteria, only 267 patients were included in the analysis. Of these, 105 received systemic corticosteroids (dexamethasone 6mg/day or equivalent) plus standard care, while the others were treated with standard care.
During hospitalization, each patient had a consecutive nasopharyngeal swab RT-PCR test every other day. The admission date was used as the starting time-point for the viral clearance process. Negative conversion time of SARS-CoV-2 was defined as the interval between first positive SARS CoV-2 and the first of two consecutive negative virus tests (sampling interval at least 1 day). A delayed negative conversion was defined as a time length of nasopharyngeal swab RT-PCR negativization longer than 20 days.
The study was conducted in accordance with the Declaration of Helsinki of the World Medical Association and was approved by the Ethics Committee of the Azienda Ospedaliera dei Colli, Naples, Italy, that also waived the need for informed consent.
Patients were divided into two groups based on the median duration of viral shedding: those that shedded the virus less than 20 days and those that shedded virus more than 20 days. We used the Mann–Whitney U test to compare the differences between the two groups for quantitative variables, the Pearson's chi-square and Fisher's exact tests to assess the different characteristics in the two groups, and the Receiver Operating Characteristic (ROC) curve to assess the potential of the multivariable logistic regression model. The corresponding area under the curve (AUC) was also calculated. In addition, we used the Kaplan–Meier survival analysis to estimate the cumulative rate of SARS-CoV-2 RNA negative conversion, multivariable logistic regression to identify potential factors associated with the duration of viral shedding, and the Cox proportional hazards regression model to evaluate the hazard ratio (HR) of each variable for the duration of viral positivity to SARS-CoV-2, and significant risk factors whose HR was further adjusted by covariate analysis.
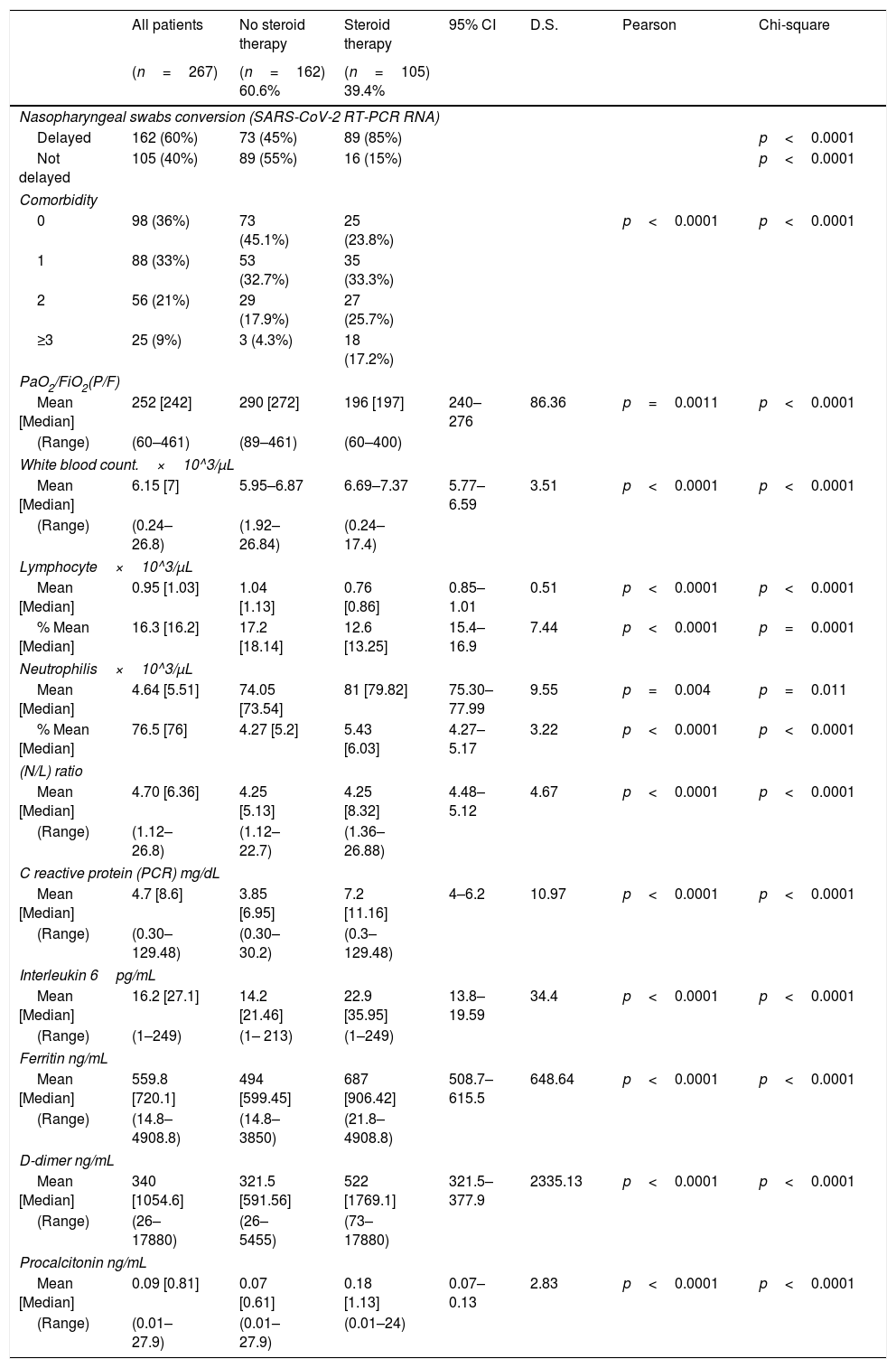
The demographics and laboratory findings on admission of patients included in the analysis are reported in Table 1.
Clinical characteristics and laboratory findings on admission in the study population.
| All patients | No steroid therapy | Steroid therapy | 95% CI | D.S. | Pearson | Chi-square | |
|---|---|---|---|---|---|---|---|
| (n=267) | (n=162) 60.6% | (n=105) 39.4% | |||||
| Nasopharyngeal swabs conversion (SARS-CoV-2 RT-PCR RNA) | |||||||
| Delayed | 162 (60%) | 73 (45%) | 89 (85%) | p<0.0001 | |||
| Not delayed | 105 (40%) | 89 (55%) | 16 (15%) | p<0.0001 | |||
| Comorbidity | |||||||
| 0 | 98 (36%) | 73 (45.1%) | 25 (23.8%) | p<0.0001 | p<0.0001 | ||
| 1 | 88 (33%) | 53 (32.7%) | 35 (33.3%) | ||||
| 2 | 56 (21%) | 29 (17.9%) | 27 (25.7%) | ||||
| ≥3 | 25 (9%) | 3 (4.3%) | 18 (17.2%) | ||||
| PaO2/FiO2(P/F) | |||||||
| Mean [Median] | 252 [242] | 290 [272] | 196 [197] | 240–276 | 86.36 | p=0.0011 | p<0.0001 |
| (Range) | (60–461) | (89–461) | (60–400) | ||||
| White blood count.×10^3/μL | |||||||
| Mean [Median] | 6.15 [7] | 5.95–6.87 | 6.69–7.37 | 5.77–6.59 | 3.51 | p<0.0001 | p<0.0001 |
| (Range) | (0.24–26.8) | (1.92–26.84) | (0.24–17.4) | ||||
| Lymphocyte×10^3/μL | |||||||
| Mean [Median] | 0.95 [1.03] | 1.04 [1.13] | 0.76 [0.86] | 0.85–1.01 | 0.51 | p<0.0001 | p<0.0001 |
| % Mean [Median] | 16.3 [16.2] | 17.2 [18.14] | 12.6 [13.25] | 15.4–16.9 | 7.44 | p<0.0001 | p=0.0001 |
| Neutrophilis×10^3/μL | |||||||
| Mean [Median] | 4.64 [5.51] | 74.05 [73.54] | 81 [79.82] | 75.30–77.99 | 9.55 | p=0.004 | p=0.011 |
| % Mean [Median] | 76.5 [76] | 4.27 [5.2] | 5.43 [6.03] | 4.27–5.17 | 3.22 | p<0.0001 | p<0.0001 |
| (N/L) ratio | |||||||
| Mean [Median] | 4.70 [6.36] | 4.25 [5.13] | 4.25 [8.32] | 4.48–5.12 | 4.67 | p<0.0001 | p<0.0001 |
| (Range) | (1.12–26.8) | (1.12–22.7) | (1.36–26.88) | ||||
| C reactive protein (PCR) mg/dL | |||||||
| Mean [Median] | 4.7 [8.6] | 3.85 [6.95] | 7.2 [11.16] | 4–6.2 | 10.97 | p<0.0001 | p<0.0001 |
| (Range) | (0.30–129.48) | (0.30–30.2) | (0.3–129.48) | ||||
| Interleukin 6pg/mL | |||||||
| Mean [Median] | 16.2 [27.1] | 14.2 [21.46] | 22.9 [35.95] | 13.8–19.59 | 34.4 | p<0.0001 | p<0.0001 |
| (Range) | (1–249) | (1– 213) | (1–249) | ||||
| Ferritin ng/mL | |||||||
| Mean [Median] | 559.8 [720.1] | 494 [599.45] | 687 [906.42] | 508.7–615.5 | 648.64 | p<0.0001 | p<0.0001 |
| (Range) | (14.8–4908.8) | (14.8–3850) | (21.8–4908.8) | ||||
| D-dimer ng/mL | |||||||
| Mean [Median] | 340 [1054.6] | 321.5 [591.56] | 522 [1769.1] | 321.5–377.9 | 2335.13 | p<0.0001 | p<0.0001 |
| (Range) | (26–17880) | (26–5455) | (73–17880) | ||||
| Procalcitonin ng/mL | |||||||
| Mean [Median] | 0.09 [0.81] | 0.07 [0.61] | 0.18 [1.13] | 0.07–0.13 | 2.83 | p<0.0001 | p<0.0001 |
| (Range) | (0.01–27.9) | (0.01–27.9) | (0.01–24) | ||||
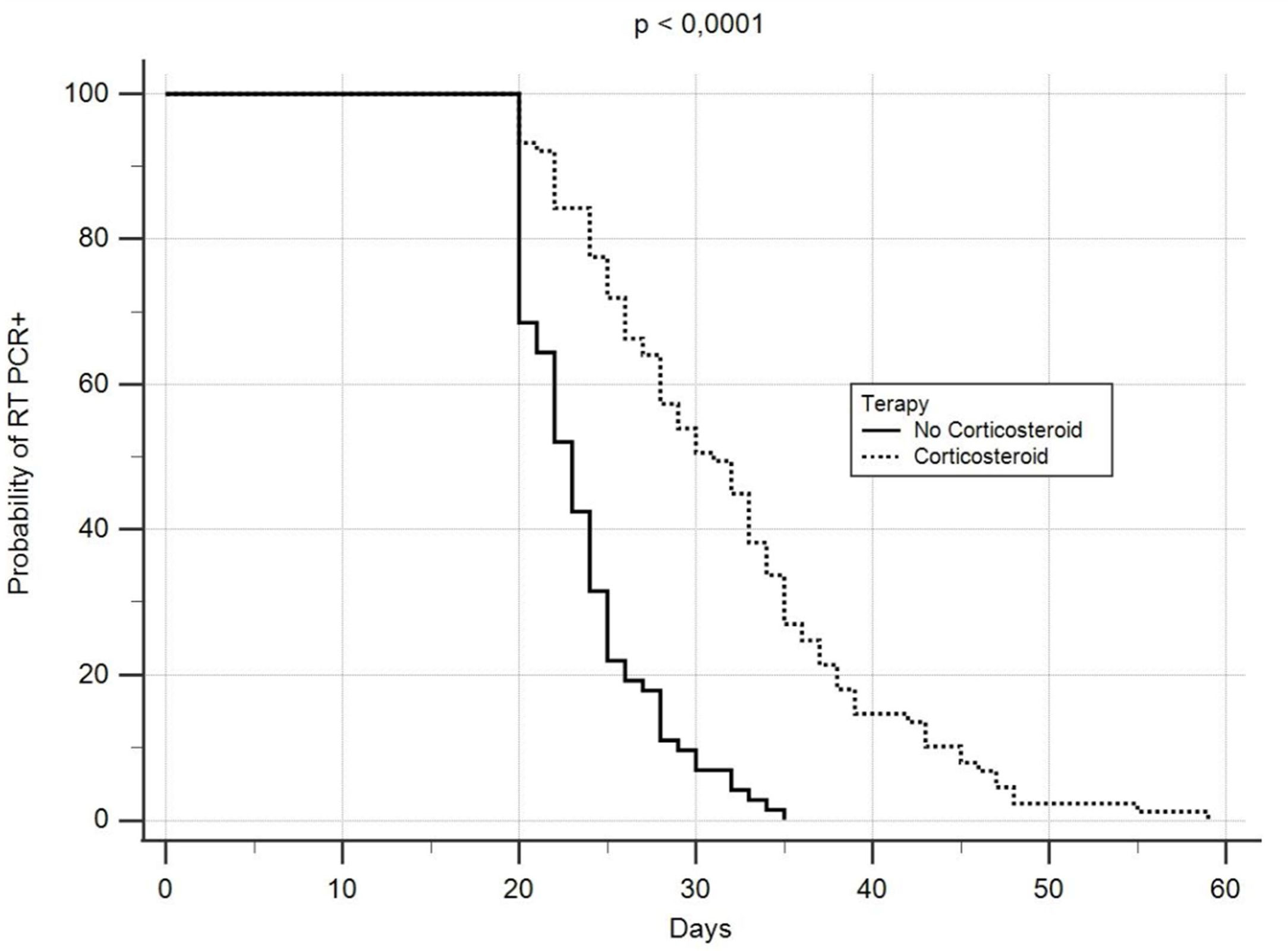
The median time to negative RT-PCR conversion was 20 days (range: 7–59), while the median length of hospitalization was 24 days (range: 7–136). However, the rate of negative RNA conversion within 20 days among all patients was 39.3%, while 67.7% of them showed a longer time to negative viral RNA conversion. There was a significant correlation between corticosteroid treatment and delay in negative SARS-CoV-2 RNA conversion (Fig. 1), with a delayed RT-PCR nasopharyngeal swab negativity reported in 89 of the 105 patients who received systemic corticosteroids. A PaO2 to FiO2 ratio<247 appeared to predict a delay in negative viral RNA conversion. Forty-five% of patients not treated with corticosteroids remained RT-PCR positive for more than 20 days, and had a significant association between delayed time to negative conversion of SARS-CoV-2 RNA and lymphopenia.
Our data suggest that corticosteroids induce beneficial effects in severe COVID-19 patients, but can also delay viral clearance. It must be highlighted that RT-PCR positivity after recovery does not necessarily imply the presence of a viable or transmissible virus,5,6 but long viral shedding times are important for determining hospital discharge, discontinuation of quarantine and the effect of antiviral treatment for COVID-19.7,8 Furthermore, it could increase the risk of secondary infection with worse outcomes, especially in those with impaired immune systems.9
Our findings fully match the trend recorded in a recent systematic review and meta-analysis in which the average viral shedding time in patients receiving corticosteroid treatment was 28.3 days, which was longer than in those without corticosteroid treatment (16.2 days) although no statistically significant difference was found.7 It has also been documented that corticosteroids delay viral clearance mainly in those taking a high or medium dose.10 Furthermore, a high risk of viral clearance delay seems to be significantly associated with late use rather than early use of corticosteroids.10
This study suffers from some limitations which are not only its single-center setting and the use of retrospective data, but also the lack of measurement of viral loads, which might have influenced the duration of RT-PCR negativization of the nasopharyngeal swab, and the non-detection of the virus from the viral culture, which do not allow us to determine whether RT-PCR positive patients had infectivity. In addition, we did not study T lymphocyte subsets.
At the time of admission, there were significantly fewer lymphocytes and more neutrophils in the blood of subjects who would be treated with corticosteroids than in those in whom such treatment was not administered. An increased neutrophil-to-lymphocyte ratio and decreased CD4+ T-cells are significant risk factors for prolonged viral shedding of the respiratory tract.11 In addition, corticosteroids may inhibit pulmonary inflammation and alleviate possible immune-mediated lung damage, but also inhibit the systemic immune response dominated by the T-cell response, resulting in delayed virus clearance.12 Unfortunately, our data do not allow us to determine whether the decrease in lymphocyte numbers was due to a decrease in CD4+ T cells.
It is noteworthy that our corticosteroid-treated patients had higher levels of D-dimer than those not treated with corticosteroids. There is uncertainty as to whether D-dimer levels may influence the association between corticosteroid treatment and delayed viral clearance in COVID-19 patients. However, it has been reported that D-dimer values>1000ng/mL on admission independently predict prolonged SARS-CoV-2 RNA shedding.13
The results of these studies support our view that it is essential to determine which patient can be treated with a corticosteroid, when treatment should be initiated, which corticosteroid is preferable for that specific patient, and how long it should be administered when managing an SARS-CoV-2 infection. Furthermore, it is crucial to always consider all aspects of the risk-benefit ratio when treating a patient with COVID-19 with a corticosteroid.
AuthorshipRP and AB participated in the study's conception and design and supervised the study.
MC wrote the manuscript.
AM, VD’A performed the statistical analysis.
RP, FS, EM, VE, RP, FF, VD’A and the Cotugno COVID team participated in the data collection.
All the authors contributed to data interpretation, critically revised the manuscript and approved the final version for publication.
MC and AB are the guarantors of the paper.
Funding sourcesNo funding or economic supports were received for this study.
Conflicts of interestsThe authors have no conflicts of interests to declare.
Cotugno COVID team: A. De Rosa1, A. Pontarelli1, V. Iodice3, M. Sardo3, S. Mascolo4, A.M. Rossomando4, V. Bianco5, F. Martucci5