Chronic bronchial infection (CBI) is a frequent and challenging condition in patients with chronic respiratory diseases such as bronchiectasis and chronic obstructive pulmonary disease (COPD). Chronic colonization by potentially pathogenic microorganisms (PPMs) contributes to persistent inflammation, impaired mucociliary clearance, and increased risk of exacerbations, hospital admissions, and mortality [1–4]. Inhaled antibiotics (IA) have demonstrated clinical benefit, particularly in the presence of Pseudomonas aeruginosa [4–6].
The European Respiratory Society (ERS) guidelines for the management of bronchiectasis highlight airway clearance techniques (ACTs), including manual and device-assisted approaches, as a cornerstone of non-pharmacological treatment in patients with bronchiectasis or impaired mucus clearance [6–8]. ACTs are safe and have been associated with improved mucociliary clearance, reduction of symptoms, enhanced quality of life, and fewer exacerbations and hospitalizations [7–13]. Nevertheless, the role of ACTs as an adjunct to inhaled antibiotic therapy in reducing exacerbations and hospital admissions remains to be fully elucidated. The aim of this study was to evaluate changes in exacerbation frequency and hospital admissions among patients with CBI receiving IA therapy, according to their adherence to regular ACTs.
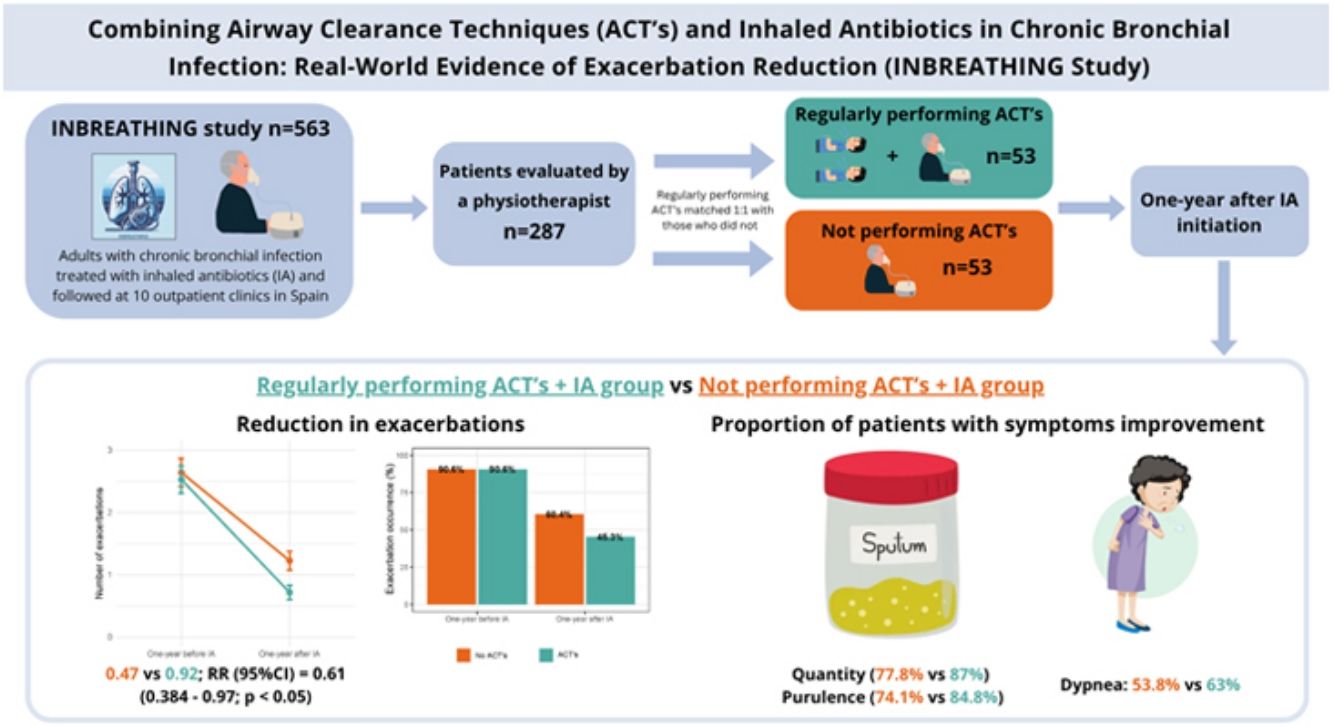
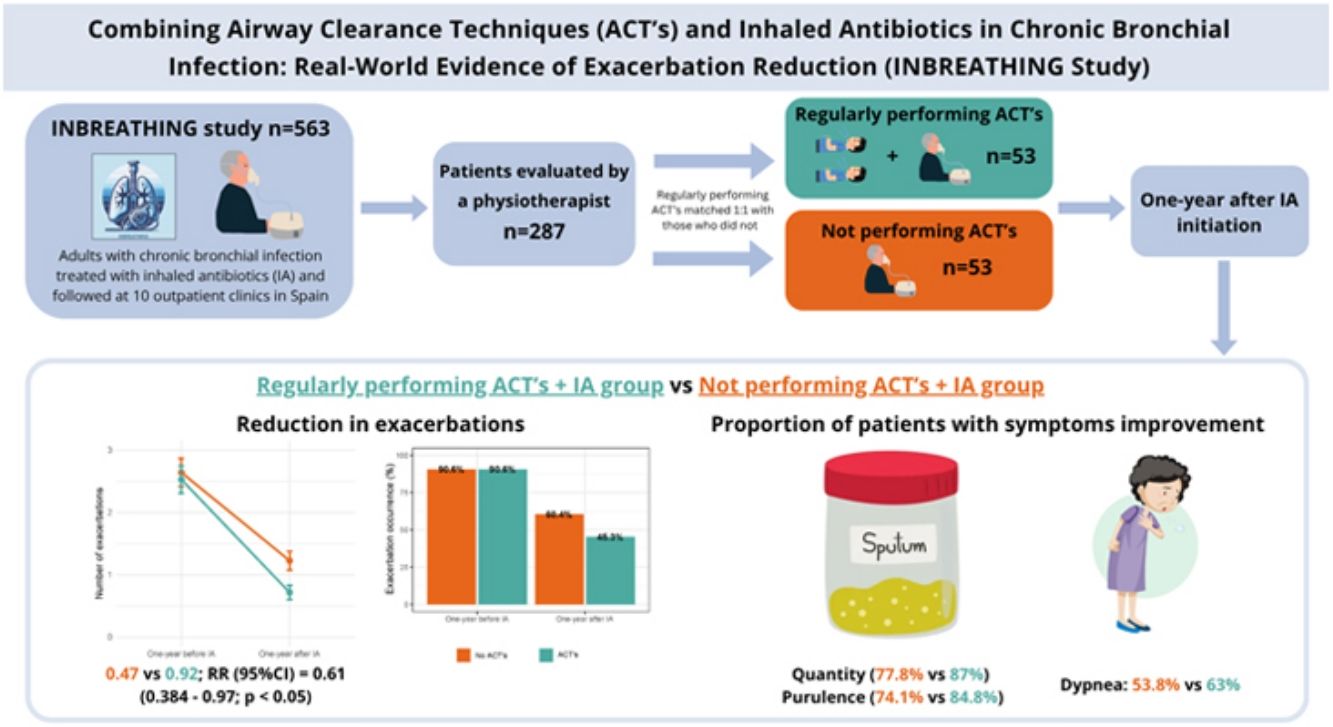
A retrospective, multicenter, observational cohort study was conducted using data from the INBREATHING cohort, which includes 563 adult patients with CBI treated with IA and followed at specialized outpatient clinics with expertise in inhaled antibiotic therapy across ten Spanish hospitals between January 2018 and June 2025. Among them, patients evaluated by a specialized respiratory physiotherapist (n=287) were selected. Those regularly performing ACTs were matched 1:1 with those who did not, using propensity score matching with a caliper of 0.2, adjusted for age, number of exacerbations in the previous year, COPD diagnosis, pneumonia, bronchiectasis, Pseudomonas aeruginosa colonization, inhaled therapy, and FEV1 (n=53 per group). CBI was diagnosed according to national guideline criteria [1,2].
Referral to physiotherapy was made by the treating pulmonologist according to clinical judgment. Adherence to ACTs was assessed retrospectively based on patient self-report, as documented by pulmonologists and/or respiratory physiotherapists in the medical records at the time of IA initiation and during the one-year follow-up visits. No specific percentage threshold was applied, and exact adherence rates or details of the specific techniques used were not systematically recorded. In general, physiotherapists recommended performing ACTs twice daily, preferably prior to IA administration. Baseline clinical, functional, and microbiological data were collected at the time of IA initiation. These included sociodemographic characteristics, underlying respiratory disease, identified PPMs, type of IA used, and the number of exacerbations and hospital admissions during the 12 months preceding IA initiation. Follow-up at one year assessed changes in symptoms, exacerbation frequency, and hospital admissions. Symptomatic changes were pragmatically defined as a reduction in sputum volume compared with baseline, a shift to less purulent sputum, and/or an improvement in dyspnea severity as documented in clinical evaluations. Statistical analysis included descriptive statistics. Group comparisons were performed using t-tests or Chi-squared tests, as appropriate. The primary outcome—number of exacerbations over one year—was analyzed using a zero-inflated Poisson mixed effects model. All analyses were conducted using R version 4.0.1.
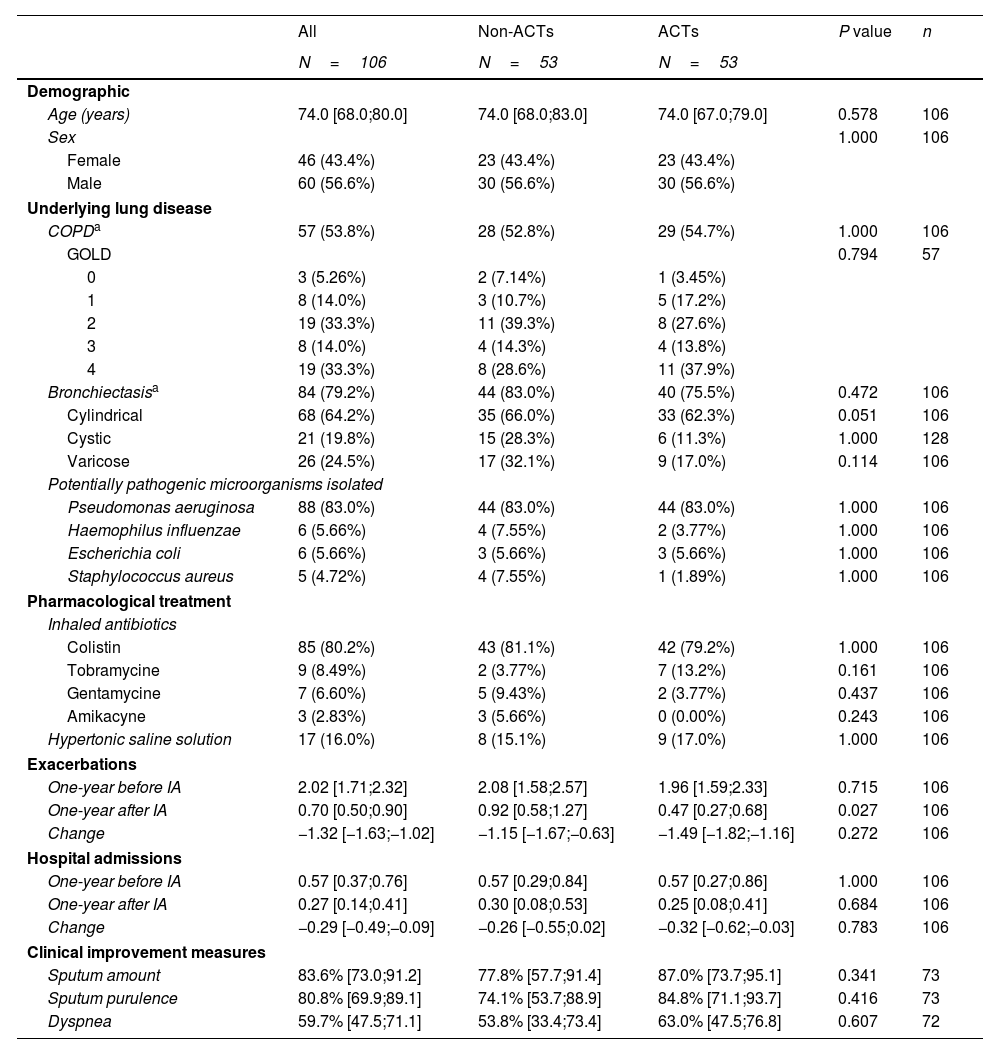
A total of 106 patients were included, predominantly male (56.6%), with a median age of 74.0 years (IQR 65.0–80.0). The most prevalent underlying respiratory diseases were bronchiectasis (79.2%) and COPD (53.8%). Pseudomonas aeruginosa was the most frequently isolated pathogen (83%), and colistin was the most commonly used inhaled antibiotic (80.2%). Concomitant therapy with hypertonic saline was prescribed in 16% of patients. Baseline characteristics were comparable between groups (Table 1).
Clinical, sociodemographic and microbiological characteristics, exacerbations, hospitalizations and symptom improvement by group.
| All | Non-ACTs | ACTs | P value | n | |
|---|---|---|---|---|---|
| N=106 | N=53 | N=53 | |||
| Demographic | |||||
| Age (years) | 74.0 [68.0;80.0] | 74.0 [68.0;83.0] | 74.0 [67.0;79.0] | 0.578 | 106 |
| Sex | 1.000 | 106 | |||
| Female | 46 (43.4%) | 23 (43.4%) | 23 (43.4%) | ||
| Male | 60 (56.6%) | 30 (56.6%) | 30 (56.6%) | ||
| Underlying lung disease | |||||
| COPDa | 57 (53.8%) | 28 (52.8%) | 29 (54.7%) | 1.000 | 106 |
| GOLD | 0.794 | 57 | |||
| 0 | 3 (5.26%) | 2 (7.14%) | 1 (3.45%) | ||
| 1 | 8 (14.0%) | 3 (10.7%) | 5 (17.2%) | ||
| 2 | 19 (33.3%) | 11 (39.3%) | 8 (27.6%) | ||
| 3 | 8 (14.0%) | 4 (14.3%) | 4 (13.8%) | ||
| 4 | 19 (33.3%) | 8 (28.6%) | 11 (37.9%) | ||
| Bronchiectasisa | 84 (79.2%) | 44 (83.0%) | 40 (75.5%) | 0.472 | 106 |
| Cylindrical | 68 (64.2%) | 35 (66.0%) | 33 (62.3%) | 0.051 | 106 |
| Cystic | 21 (19.8%) | 15 (28.3%) | 6 (11.3%) | 1.000 | 128 |
| Varicose | 26 (24.5%) | 17 (32.1%) | 9 (17.0%) | 0.114 | 106 |
| Potentially pathogenic microorganisms isolated | |||||
| Pseudomonas aeruginosa | 88 (83.0%) | 44 (83.0%) | 44 (83.0%) | 1.000 | 106 |
| Haemophilus influenzae | 6 (5.66%) | 4 (7.55%) | 2 (3.77%) | 1.000 | 106 |
| Escherichia coli | 6 (5.66%) | 3 (5.66%) | 3 (5.66%) | 1.000 | 106 |
| Staphylococcus aureus | 5 (4.72%) | 4 (7.55%) | 1 (1.89%) | 1.000 | 106 |
| Pharmacological treatment | |||||
| Inhaled antibiotics | |||||
| Colistin | 85 (80.2%) | 43 (81.1%) | 42 (79.2%) | 1.000 | 106 |
| Tobramycine | 9 (8.49%) | 2 (3.77%) | 7 (13.2%) | 0.161 | 106 |
| Gentamycine | 7 (6.60%) | 5 (9.43%) | 2 (3.77%) | 0.437 | 106 |
| Amikacyne | 3 (2.83%) | 3 (5.66%) | 0 (0.00%) | 0.243 | 106 |
| Hypertonic saline solution | 17 (16.0%) | 8 (15.1%) | 9 (17.0%) | 1.000 | 106 |
| Exacerbations | |||||
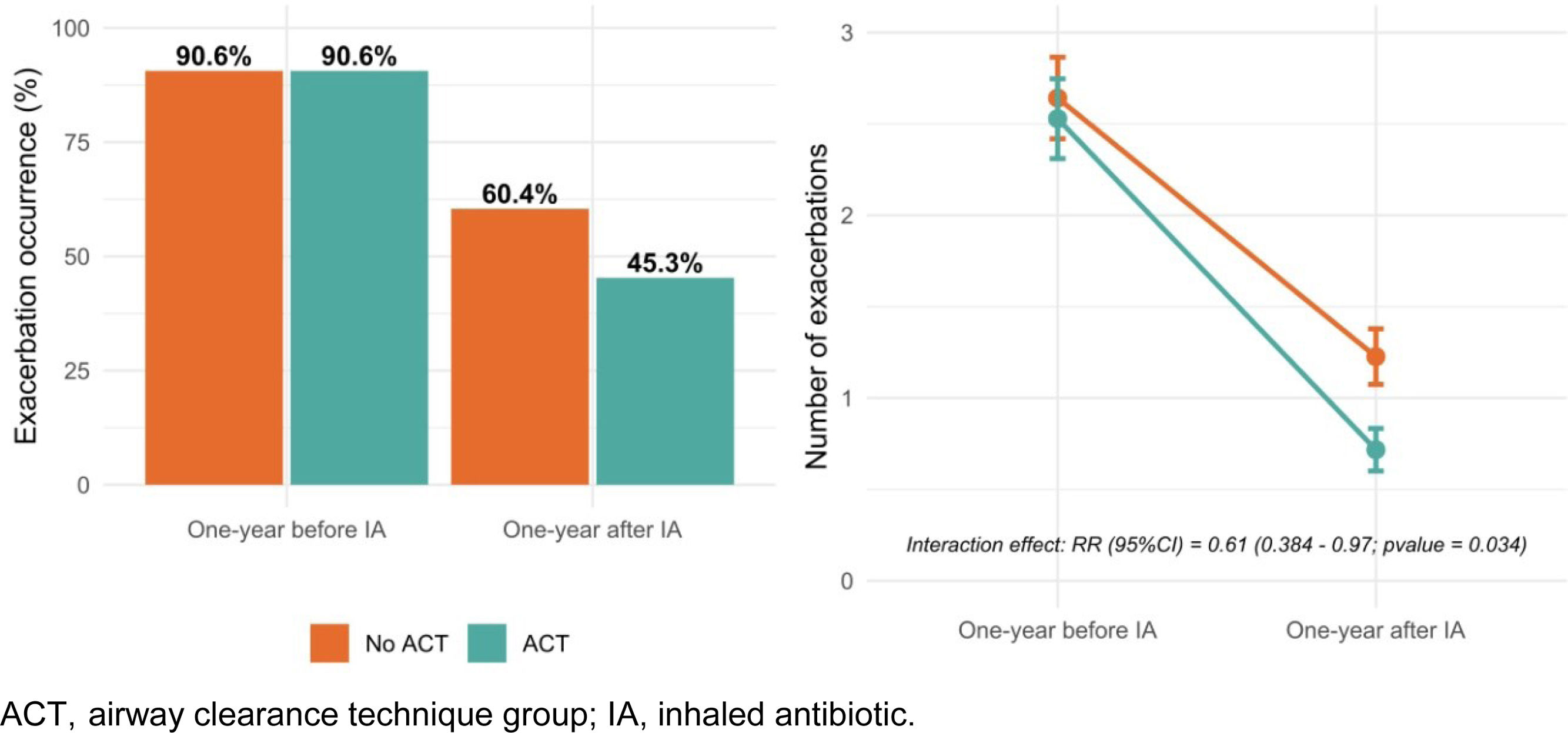
| One-year before IA | 2.02 [1.71;2.32] | 2.08 [1.58;2.57] | 1.96 [1.59;2.33] | 0.715 | 106 |
| One-year after IA | 0.70 [0.50;0.90] | 0.92 [0.58;1.27] | 0.47 [0.27;0.68] | 0.027 | 106 |
| Change | −1.32 [−1.63;−1.02] | −1.15 [−1.67;−0.63] | −1.49 [−1.82;−1.16] | 0.272 | 106 |
| Hospital admissions | |||||
| One-year before IA | 0.57 [0.37;0.76] | 0.57 [0.29;0.84] | 0.57 [0.27;0.86] | 1.000 | 106 |
| One-year after IA | 0.27 [0.14;0.41] | 0.30 [0.08;0.53] | 0.25 [0.08;0.41] | 0.684 | 106 |
| Change | −0.29 [−0.49;−0.09] | −0.26 [−0.55;0.02] | −0.32 [−0.62;−0.03] | 0.783 | 106 |
| Clinical improvement measures | |||||
| Sputum amount | 83.6% [73.0;91.2] | 77.8% [57.7;91.4] | 87.0% [73.7;95.1] | 0.341 | 73 |
| Sputum purulence | 80.8% [69.9;89.1] | 74.1% [53.7;88.9] | 84.8% [71.1;93.7] | 0.416 | 73 |
| Dyspnea | 59.7% [47.5;71.1] | 53.8% [33.4;73.4] | 63.0% [47.5;76.8] | 0.607 | 72 |
Data are presented n (%) or mean [95%CI]. ACTs, airway clearance techniques; IA, inhaled antibiotic; COPD, chronic obstructive pulmonary disease.
In the year prior to IA initiation, the mean number of exacerbations (95% CI) was 2.08 (1.58–2.57) in the non-ACT group and 1.96 (1.59–2.33) in the ACT group. One year after starting IA therapy, the mean number of exacerbations decreased to 0.92 (0.58– 1.27) in the non-ACT group and 0.47 (0.27–0.68) in the ACT group. Although both groups showed improvement, patients who regularly performed ACTs experienced a significantly greater reduction, with a rate ratio (95% CI) of 0.61 (0.38–0.97) (Fig. 1). This association was not observed for hospital admissions. Additionally, patients in the ACT group reported greater improvement in sputum quantity, purulence, and dyspnea, although these differences did not reach statistical significance (Table 1).
In this multicenter, retrospective study involving 106 participants with CBI receiving IA therapy, regular adherence to ACTs was associated with superior clinical outcomes compared to IA therapy alone. Specifically, regular ACT performance combining with IA therapy was linked to a greater reduction in exacerbation frequency and more pronounced improvements in sputum volume, purulence, and dyspnea.
Evidence on the regular use of ACTs in patients with CBI remains limited. To our knowledge, this is the first real-world study to demonstrate potential clinical benefits of regular ACTs in CBI patients treated with IA therapy. In our cohort, the most common underlying conditions were bronchiectasis (79.2%) and COPD (53.8%). International guidelines recommend ACTs in bronchiectasis [6–8,11,13,14]. In COPD, while the evidence base is more limited, recent systematic and scoping reviews support the use of ACTs in selected patients with chronic sputum production [10]. Despite these recommendations, access to specialized physiotherapy is often limited. For example, European registry study [7] reported that 52% of patients with bronchiectasis regularly used ACTs. In our study, however, only 51% of patients were evaluated by a respiratory physiotherapist, and just 18.5% reported regular ACT use—likely reflecting patient-related barriers (e.g., time burden, technique complexity, limited perceived benefit), variability in clinical practice and referral across centers, and socioeconomic factors such as education, support and comorbidities. Most published studies are small and focus on short-term outcomes. Consistent with our findings, a randomized controlled trial by Muñoz G. et al. [15] involving 44 stable bronchiectasis patients with chronic sputum production demonstrated that daily ACT use over 12 months reduced exacerbations (from 2.0 [1.0–3.25] to 1.0 [0–2.0]), while the control group showed an increase (from 1.0 [0.75–2.25] to 2.0 [1.0–3.0]). This study is one of the few with a one-year follow-up. Additionally, a prospective study by Chandrasekar et al. [16] showed that oscillating positive expiratory pressure therapy with the Acapella device significantly reduced exacerbation frequency, improved lung function (FEV1), and enhanced quality of life compared with conventional physiotherapy in patients with bronchiectasis. In COPD, follow-up periods are generally shorter. However, several randomized controlled trials [17–20] with follow-ups of up to six months have also shown that ACTs can significantly reduce exacerbation frequency and improve respiratory symptoms compared with standard medical therapy. The lack of a significant decrease in hospitalizations in our cohort is most likely explained by the already low pre-treatment hospitalization rate prior to the initiation of IA therapy.
A possible explanation for our findings is that regular performance of ACTs reduces both sputum volume and purulence [7–13], thereby lowering the bacterial load in patients with CBI receiving IA therapy. Beyond facilitating mechanical clearance, regular use of ACTs may also limit bacterial overgrowth by enhancing mucus transport and promoting the removal of hyperconcentrated, adhesive secretions that would otherwise serve as a reservoir for microbial persistence. Furthermore, by reducing the retention of inflammatory mediators such as neutrophil elastase and mucins, ACTs may help attenuate airway inflammation, and—together with improved bacterial control—contribute to greater disease stability and fewer exacerbations [7,8,13]. These results have meaningful clinical implications, underscoring the importance of incorporating regular ACTs into routine care. Furthermore, they provide a strong rationale for the design and implementation of prospective clinical trials to evaluate the efficacy of ACTs in this patient population.
This study has several limitations. The assessment of ACT impact was not a predefined objective, and adherence was evaluated through subjective, non-standardized measures based on medical records and physiotherapy notes, preventing calculation of exact adherence rates. Specific techniques and start dates of ACTs were not systematically recorded, and improvements in symptoms and sputum were defined pragmatically rather than with objective scales. Although all patients classified as adherent were already performing ACTs at the time of IA initiation, we lacked detailed information on the duration of prior adherence or on possible changes during follow-up, which prevented us from analyzing the impact of adherence history. In addition, the study did not record the exact date of each exacerbation, precluding analyses based on their timing or temporal distribution. Moreover, validated severity scores for bronchiectasis or multidimensional COPD systems (GesEPOC/GOLD) were not consistently available. Strengths include the use of real-world data from 10 Spanish hospitals, a relatively large and well-characterized sample, and a one-year follow-up, providing a pragmatic view of routine clinical practice. Importantly, this study generates a clinically relevant hypothesis and defines a translational field of research that should be addressed in future prospective studies to clarify the impact of combining ACTs with IA therapy.
In conclusion, among patients with CBI treated with IA, the regular use of ACTs in combination with IA was associated with a greater reduction in exacerbations and improved respiratory symptoms compared with IA alone. Despite its limitations, these real-world findings support the integration of ACTs into routine management of this patient population. While prospective, controlled studies are needed to confirm these results and define optimal ACT protocols, our data provide a compelling rationale for considering ACT adherence as a key component in the comprehensive care of patients with CBI receiving IA therapy.
Authors’ contributionsConceptualization (AM, IDB, JG), data curation (IDB, AS, AM), formal analysis (IDB, AS), investigation (all), methodology (AM, IDB, JG, AS, DR, GS), project administration (JG, AS, DR, GS), supervision (JG, AS, IDB, AM), writing – original draft (AM, JG, IDB, AS), and writing – review & editing (all). All authors provided final approval of the version submitted for publication.
Artificial intelligence involvementThe authors declare that no material has been partially or totally produced with the help of artificial intelligence.
Sources of supportAS was supported by Departament de Salut (Pla Estratègic de Recerca i Innovació en Salut (PERIS): SLT028/23/000191), SS was supported by Departament de Salut (Pla Estratègic de Recerca i Innovació en Salut (PERIS): SLT035/24/000025).
Conflicts of interestThe authors declare not to have any conflicts of interest that may be considered to influence directly or indirectly the content of the manuscript.