Dasatinib is a potent second-generation BCR-ABL1 tyrosine kinase inhibitor (TKI) that is used at a daily dose of 100 mg as first-line therapy in patients with maintenance-stage chronic myeloid leukemia (CML), and in patients resistant or intolerant to previous therapy.1,2
Dasatinib has been associated with the development of pleural effusion (PE), produced by different mechanisms.3 Secondary chylothorax has rarely been described in the literature.4–9
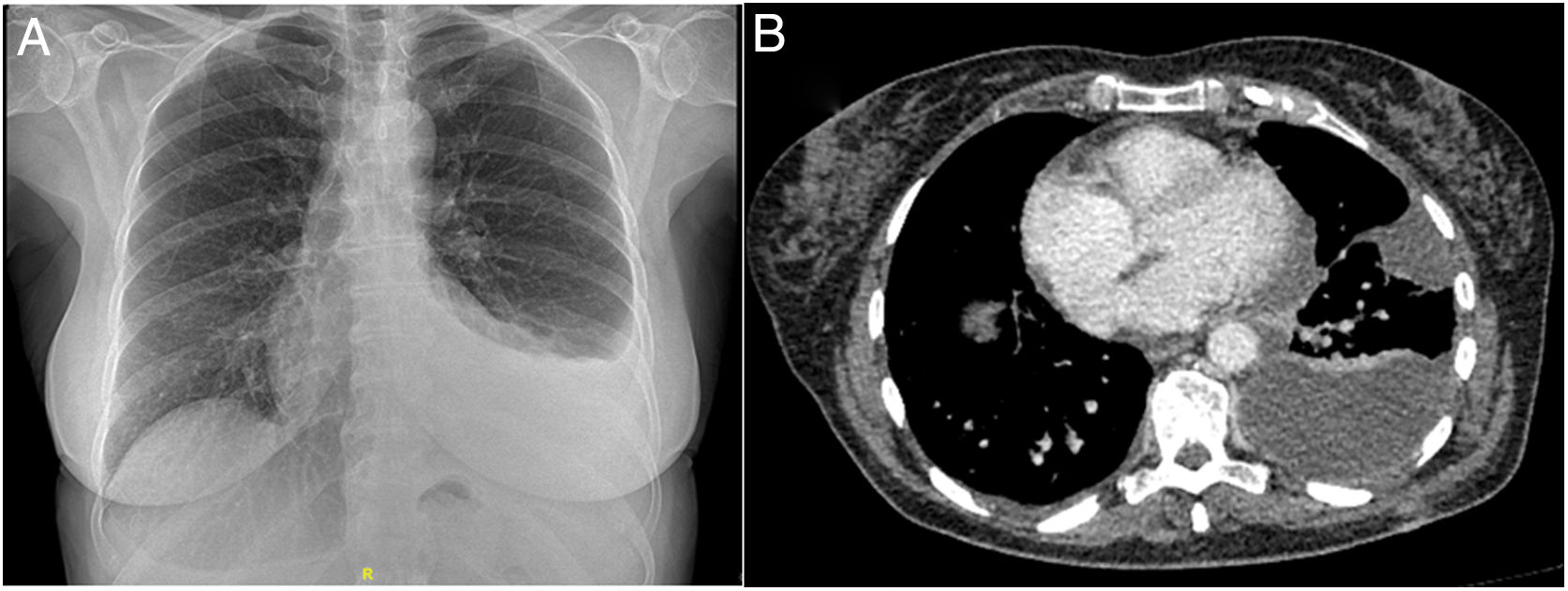
Clinical caseOur patient was a 63-year-old woman who attended the emergency department with a 2-month history of dyspnea on exertion and in left lateral decubitus, without fever, chest pain, or constitutional symptoms. She had a previous diagnosis of CML, and had been receiving oral dasatinib 100 mg/day for a year. On examination, her breathing was normal and her general status was good; BP: 146/80 mmHg; temperature: 35.5°C; heart rate: 80 beats/min; SpO2: 97%. Pulmonary auscultation identified decreased breath sounds in the lower left field, with signs of PE. All other parameters were normal. The chest X-ray revealed PE in the left lower third field, with a normal cardiac silhouette (Fig. 1A).
Blood count showed 5900 leukocytes with 54% neutrophils, 25.4% lymphocytes, 7.2% eosinophils, hemoglobin 12.8 g/dl, mean corpuscular volume 90.6, platelets 24,2000, ESR 34 mm. Biochemistry, autoimmune tests and immunoglobulins were normal. Thoracentesis yielded milky-looking pleural fluid (PF), with the following values: glucose 90 mg/dl, albumin 2.30 g/dl, LDH 152 u/l, proteins 5.05 g/dl, cholesterol 106 mg/dl, triglycerides 334 mg/dl, CEA 1.2 ng/ml, rheumatoid factor < 10 IU/ml, ANA negative, leucocytes 1470/mm3, RBCs 3000/mm3, mononuclear cells 98.8%, polynuclear cells 1.2%. Cytology showed predominantly lymphoid reactive cytoarchitecture with some elements of myeloid ontogeny and no evidence of malignancy. Bacterial culture including Lowenstein was negative. Pleural biopsy revealed mesothelial hyperplasia. The echocardiogram was normal.
Dasatinib was discontinued and a chest drain was inserted, yielding 1,600 ml after 5 days of admission, and a visit was scheduled for 15 days later. Chest computed tomography (CT) 10 days after discharge showed left organizing PE (Fig. 1B). A new thoracentesis showed amber-colored PF, pH 7.55, glucose 148 mg/dl, LDH 169 u/l, cholesterol 120 mg/dl, triglycerides 81 mg/dl, proteins 5.32 g/dl, leukocytes 2874/mm3, polynuclear 31.8%, mononuclear 68.2%, red blood cells 5000/mm3; 600 ml fluid was evacuated. The patient's progress was favorable following administration of 60 mg prednisone for 15 days in a tapering regimen, and switching dasatinib to imatinib. PE resolved with no sequelae observed on chest CT a month later.
DiscussionAlthough edemas and hydrosaline retention have been associated with TKIs used to treat CML, exudative PE3 and even chylothorax have frequently been reported with dasatinib.4–9 Immune mechanisms have been implicated in its production, due to the presence of predominantly lymphocytic exudate. Alternatively, it has been proposed that PE (transudate or exudate) might also be caused by blocked T cell function at clinically relevant concentrations, affecting proliferation, activation and cytokine production, or inhibition of platelet-derived growth factor receptor ® (PDGFR-®) expressed in pericytes involved in angio-lymphangiogenesis.10 Other authors suggest that PDGF-BB and its receptor, PDGFR-®, are directly lymphangiogenic.11
The first cases of dasatinib-associated PE were described by Bergeron et al. in 2007 in a series of 40 patients, of which 9 developed respiratory symptoms, with PE in 7 cases, lymphocytic exudate in 6, some accompanied by predominantly lymphocytic alveolar infiltrates.12
Risk factors include age and advanced disease, cardiac and autoimmune disorders, preexisting hypertension, hypercholesterolemia, skin rash, dose and time of administration, and lymphocytosis.3–8 Correlations with minimum plasma levels, drug exposure, duration and response to treatment have also been suggested but not confirmed.
The frequency, risk factors, and outcomes associated with PE were assessed in a pooled population of 11 trials that included 2712 patients with CML and acute lymphoblastic leukemia treated with dasatinib. PE developed in 6%–15% of at-risk patients annually. With a minimum follow-up of 5–7 years, drug-related PE occurred at a rate of 28 %–33%. In multivariate analysis, age was the main risk factor. Despite PE, the overall response to dasatinib, progression-free survival, and survival were similar in patients who developed PE and in those who did not.3
In an experimental study in rats treated with dasatinib for 5 weeks, PE appeared with rapid and reversible increase in the paracellular permeability of monolayers of pulmonary endothelial cells, resulting in increased passage of macromolecules, loss of endothelial cadherin (primary cell adhesion molecule), rupture of cell bonds, and development of actin stress fibers. These results were replicated in human umbilical and venous endothelial cells, confirming a decrease in endothelial resistance. This increase in endothelial permeability is a mechanism dependent on reactive oxygen species (ROS) in vitro and in vivo.13
Chylothorax is defined as a turbid PE with triglycerides > 110 mg/dl and cholesterol levels < 200 mg/dl, which gives it its characteristic color.14 Chylothorax is associated with multiple etiologies, but dasatinib is the only drug known to be associated with this adverse effect. Chylothorax has been reported in 14 cases, including our patient.4,15 Our case is the first in the literature to show the biochemical change from chylothorax to lymphocytic PE as an expression of improved permeability before resolution, after discontinuing treatment.
Although treatment discontinuation will resolve symptoms, given the therapeutic benefit of dasatinib, dose reduction rather than complete discontinuation has been proposed, although this still needs to be demonstrated. Dasatinib may be temporarily suspended until chest drainage and supportive measures achieve symptomatic improvement, and resumed at reduced dose, although steroids, pleurodesis, and even thoracic duct ligation have also been recommended as in our case.
Please cite this article as: Molina V, Vañes S, Castelló C, Chiner E. Quilotórax espontáneo secundario a dasatinib. Arch Bronconeumol. 2020;56:599–601.