The question of whether obstructive sleep apnea (OSA) is a modifiable cardiovascular (CV) risk factor has emerged very early in the history of this syndrome. Almost 35 years ago, Partinen et al. found that severe OSA patients radically treated by tracheostomy had lower all-cause and CV mortality rates at 5 years than those conservatively treated with weight-loss, despite lower mean apnea-index and body mass index in the conservative group.1 With the advent of continuous positive airway pressure (CPAP) which quickly became the reference therapy of moderate-to-severe OSA, observational clinic-based studies have subsequently shown that adequate CPAP use (usually defined by an average nightly use of at least 4h) was associated with lower rates of CV events and death from any CV causes compared to untreated or non-adherent patients.2,3 It has also been shown that CPAP therapy improves several factors potentially implicated in the pathophysiology of OSA-associated CV diseases such as elevated blood pressure, endothelial dysfunction, and systemic inflammation.4 It was therefore deemed necessary to confirm the beneficial effect of CPAP on CV outcomes by randomized controlled trials (RCTs), and research teams that have met the challenge should be commended.4–6 To circumvent ethical and economical obstacles, RCTs were performed in minimally symptomatic patients with overt CV disease recruited from cardiology or neurology clinics, who could be expected to have a high incidence of new CV events. Unfortunately, we have now 3 concordant RCTs showing no effect of CPAP therapy in secondary prevention on major adverse CV events (MACEs).4–6 However, these studies were characterized by low levels of CPAP usage [2.8±2.7 and 3.3±2.3h/night]4,6 and sensitivity analyses have suggested a CV protection in adherent users, predominantly driven by stroke risk reduction.4,5
Assessing the impact of OSA therapies in real life conditions offers several advantages including the ability to enroll large samples of symptomatic patients with a wide range of clinical phenotypes and OSA severity, longer follow-up durations, and lower costs. Patients attending sleep centers for clinical suspicion of OSA are likely to be diagnosed according to clinical practice guidelines and to adhere to the therapy. The findings of large real-life cohort studies are likely to be generalizable to the entire OSA population (i.e., high external validity). Furthermore, as CPAP adherence is collected daily by remote monitoring, it is possible to assess a dose–response association between device usage and outcomes, and to consider non-adherent patients as a control group. However, the major bias in observational real life studies, is that the attribution to the group of treated or controls is not random and could depend on unmeasured covariates.7 RCTs are considered as providing the most rigorous evidence of treatment's effectiveness as randomization balances participant characteristics between the groups allowing attribution of any differences in outcome to the study intervention (i.e., high internal validity). However, RCTs are not free from limitations, some of which are particularly relevant in the field of OSA. A major limitation is the low external validity. Indeed, it has been recently shown that less than 10% of patients diagnosed with OSA in routine practice met the eligibility criteria to participate in one of the 3 RCTs evaluating the impact of CPAP on CV risk.8 Furthermore, RCTs have included minimally symptomatic patients with previous history of CV diseases in whom OSA seems to have limited effects on incident CV events.9,10 Low CPAP adherence is also a major parameter to take into account in the interpretation of RCTs. Indeed, using CPAP 3 or 4h from the beginning of the sleep period might leave 75% of untreated REM obstructive events that were found to be strongly associated with adverse CV outcomes.11
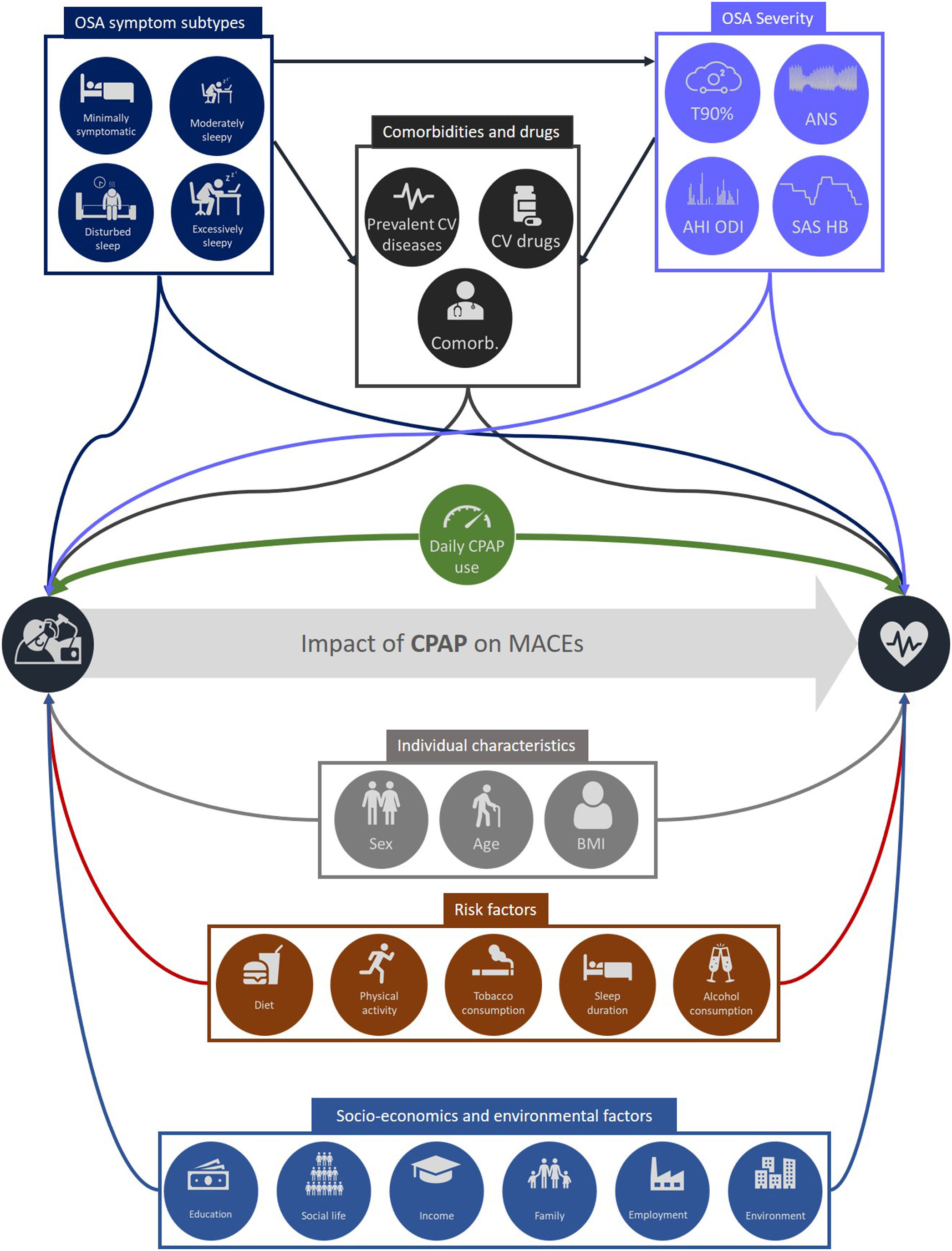
Although the minimal nightly duration of CPAP usage required to normalize functioning remains unclear, a dose–response relationship has been consistently highlighted between CPAP adherence and improvements in sleepiness, quality of life and different health outcomes such as memory, blood pressure, CV morbidity and mortality.12 However, clinicians must be careful when interpreting observational data linking PAP adherence with health outcomes, a widespread mistake being to confuse the concepts of association and causality.7 As shown in Fig. 1, many measured covariates such as anthropomorphic data, comorbidities, smoking habits, alcohol consumption and OSA severity, are relevant to the assessment of CV outcomes in the context of CPAP adherence. Additional covariates that not are routinely captured in health care data set should be also taken into account including socio-environmental factors, diet, exercise, and sleep duration which is linked both to nightly CPAP use and to CV risk.13 The healthy adherer bias is also particularly relevant to comparison of health outcomes between patients with OSA who are adherent and those who are non- or less adherent to CPAP therapy.14 The healthy adherer effect arises when patients who adhere to a therapy are more likely to engage in other healthy behaviors than their non-adherent counterparts. Patients who are adherent to statins are also more adherent to screening procedures such as eye examinations, fecal occult blood testing and prostate specific antigen testing.15 In a meta-analysis of 8 RCTs, non-adherence to placebo was associated with a 1.79-fold increase in mortality.16
Illustration of covariates that are relevant to the assessment of cardiovascular (CV) outcomes in the context of continuous positive airway pressure (CPAP) adherence. Abbreviations: OSA: obstructive sleep apnea; Comorb: comorbidities; AHI: apnea–hypopnea index; ODI: oxygen desaturation index; T90: percentage of sleep (or recording) time with oxygen saturation <90%; SAS HB: sleep apnea specific hypoxic burden; ANS: autonomic nervous system; MACEs: major adverse cardiovascular events; BMI: body mass index.
In order to capture the healthy adherer effect, the adherence to chronic CV active medications was entered as a covariate in a recent study from our group evaluating the association between CPAP adherence and incident MACEs within the Pays de la Loire Sleep Cohort.17 There was a significant association of CPAP adherence with the medication possession ratio for lipid lowering and antihypertensive drugs. However, the association of daily CPAP use with MACEs remained significant after adjusting demographics, socioeconomic status, comorbidities, alcohol intake, tobacco consumption, and CV drugs refill. Patients using CPAP at least 6h/night had a ≈25% reduction in incident MACEs when compared to non-adherent subjects. In line with previous reports, the association between CPAP adherence and MACEs was stronger in patients without overt CV disease at diagnosis, and those belonging to the excessive sleepy symptom subtype.9,10
Whether CPAP therapy of OSA has a cardio-protective effect is probably one of the questions that will never be answered by RCTs, due to obvious ethical obstacles. Observational studies suggest that this effect preferentially applies to excessively sleepy patients with a high level of CPAP adherence. Clinical management of OSA should therefore target symptomatic patients as a priority, and include support programs to increase CPAP adherence. In contrast, there is limited evidence to support systematic screening for sleep-disordered breathing in asymptomatic subject at high CV risk.18 Further real-life cohort studies enriched with additional covariates (diet, physical activity, CV drug adherence, …), measured at baseline and during follow-up, should help to better elucidate the complex relationship between CPAP adherence and CV risk, taking into account the general healthy adherer effect. Novel metrics of OSA-related hypoxic burden and autonomic nervous system dysfunction could, better than the apnea–hypopnea index, capture the physiological consequences of OSA, and identify patients with a greater CV benefit of CPAP therapy.17,19 Causal inference approaches for longitudinal data represent a promising strategy to control the influence of confounders.7
Conflict of InterestsThe authors state that they have no conflict of interests.