Hematological malignancies (HM) are a known cause of bronchiectasis, yet this association has rarely been described in detail.1 In particular, no prior studies have assessed the prevalence of bronchiectasis among subjects with HM.
Chronic lymphocytic leukemia (CLL) is associated with recurrent respiratory infections.2–4 We aimed to assess the presence and clinical significance of bronchiectasis in subjects with CLL, assuming that a significant proportion of CLL patients would suffer from bronchiectasis.
This retrospective cohort study included all adult (≥18-years-old) subjects with CLL who were treated in the Hematology Clinic between January 1st, 2000 and June 30th, 2018, and had at least one available chest CT, performed after the diagnosis of CLL.
Follow-up began at the date of CLL diagnosis (day 0) and ended at February 29th, 2020, or the patient's day of death.
All CT scans were reviewed by a thoracic radiologist specifically for study purposes in a blinded fashion, and subjects were categorized into one of two groups: with bronchiectasis or without (controls). Data were extracted from medical records, including disease stage at diagnosis and during follow-up (according to Rai method, Supplementary Table S1) and therapies for CLL. Laboratory data were collected both at the time of CLL diagnosis, and also the “worst” measurement for each variable since diagnosis: such as lowest hemoglobin or immunoglobulin levels, yet highest lymphocyte count or lactate dehydrogenase levels. We also collected data of lower respiratory tract cultures results.
Study groups were compared to assess possible risk factors for the presence of bronchiectasis, for overall mortality and mortality rates over time, and for morbidity outcomes such as unplanned hospital admissions, courses of antibiotics, and cultures positivity for resistant bacteria.
The study population included 203 subjects (Supplementary Fig. S1). Mean age at CLL diagnosis 65.3±10.3 years, 36.5% women. Average follow-up was 9.9±5.9 years. After reviewing the CT scans, bronchiectasis was identified in 88 subjects (43.3%).
Baseline variables were comparable between the study groups, including Rai stages at diagnosis and during follow-up (Supplementary Table S2 and Fig. S2). Two-third of subjects (135/203) required therapy for CLL. Therapy with venetoclax was significantly more common among the bronchiectasis group (11.4% vs. 2.6%, p=0.012). The median number of treatment lines, and treatment lines with anti-CD20 medications (rituximab or obinutuzumab) were numerically higher in subjects with bronchiectasis (Supplementary Table S2). The rates of other CLL-specific therapies were similar between groups.
More subjects from the bronchiectasis group had developed low levels of IgG (<500mg/dL) or IgA (<50mg/dL). They were also more tended to develop severe neutropenia (<500cells/μL) during follow-up (Supplementary Table S2).
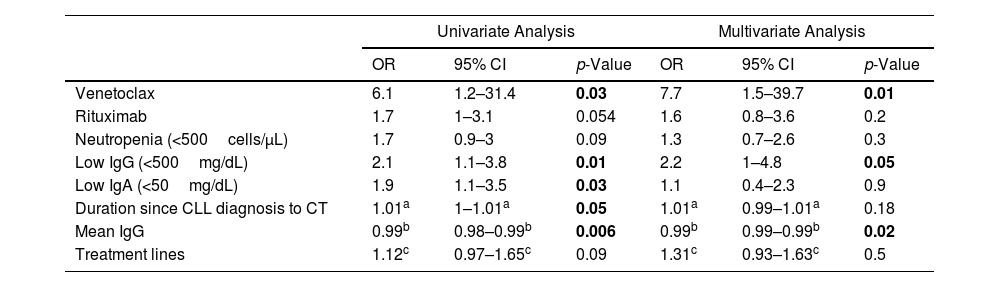
Mean IgG levels were inversely associated with bronchiectasis risk (p=0.006). Additional predictors of bronchiectasis included developing low levels of IgG or IgA during follow-up, and therapy with venetoclax. Treatment with rituximab, number of CLL treatment lines, and severe neutropenia were also associated with bronchiectasis risk, yet those associations were not statistically significant. Treatment with venetoclax (OR=7.7, p=0.014) and lower mean levels of total IgG (p=0.021) remained significantly associated with the risk for bronchiectasis in a multivariate regression model (Table 1).
Predictors of Bronchiectasis.
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Venetoclax | 6.1 | 1.2–31.4 | 0.03 | 7.7 | 1.5–39.7 | 0.01 |
| Rituximab | 1.7 | 1–3.1 | 0.054 | 1.6 | 0.8–3.6 | 0.2 |
| Neutropenia (<500cells/μL) | 1.7 | 0.9–3 | 0.09 | 1.3 | 0.7–2.6 | 0.3 |
| Low IgG (<500mg/dL) | 2.1 | 1.1–3.8 | 0.01 | 2.2 | 1–4.8 | 0.05 |
| Low IgA (<50mg/dL) | 1.9 | 1.1–3.5 | 0.03 | 1.1 | 0.4–2.3 | 0.9 |
| Duration since CLL diagnosis to CT | 1.01a | 1–1.01a | 0.05 | 1.01a | 0.99–1.01a | 0.18 |
| Mean IgG | 0.99b | 0.98–0.99b | 0.006 | 0.99b | 0.99–0.99b | 0.02 |
| Treatment lines | 1.12c | 0.97–1.65c | 0.09 | 1.31c | 0.93–1.63c | 0.5 |
Statistically significant p-values are presented in bold.
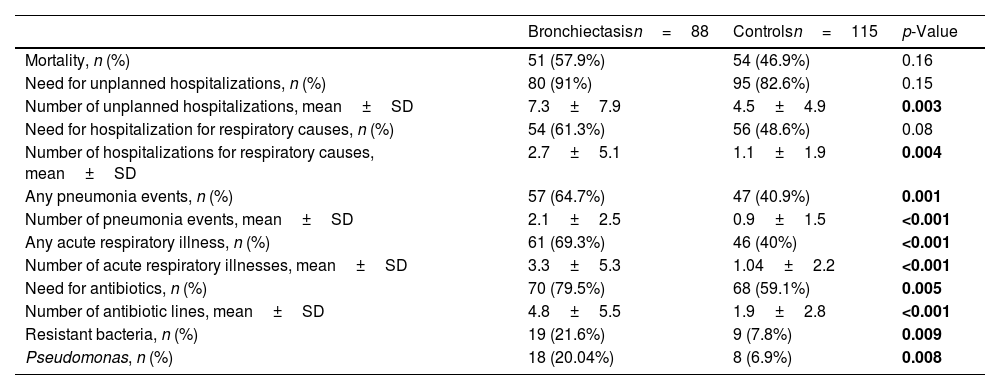
Most subjects in both groups required unplanned hospitalization. Bronchiectasis was associated with increased morbidity, including more unplanned hospital admissions, acute respiratory illnesses and pneumonia events, and more antibiotic courses. Bronchiectasis was also a risk factor for resistant bacteria in lower respiratory tract cultures, including Pseudomonas aeruginosa (Table 2).
Comparison of Outcomes Between Study Groups.
| Bronchiectasisn=88 | Controlsn=115 | p-Value | |
|---|---|---|---|
| Mortality, n (%) | 51 (57.9%) | 54 (46.9%) | 0.16 |
| Need for unplanned hospitalizations, n (%) | 80 (91%) | 95 (82.6%) | 0.15 |
| Number of unplanned hospitalizations, mean±SD | 7.3±7.9 | 4.5±4.9 | 0.003 |
| Need for hospitalization for respiratory causes, n (%) | 54 (61.3%) | 56 (48.6%) | 0.08 |
| Number of hospitalizations for respiratory causes, mean±SD | 2.7±5.1 | 1.1±1.9 | 0.004 |
| Any pneumonia events, n (%) | 57 (64.7%) | 47 (40.9%) | 0.001 |
| Number of pneumonia events, mean±SD | 2.1±2.5 | 0.9±1.5 | <0.001 |
| Any acute respiratory illness, n (%) | 61 (69.3%) | 46 (40%) | <0.001 |
| Number of acute respiratory illnesses, mean±SD | 3.3±5.3 | 1.04±2.2 | <0.001 |
| Need for antibiotics, n (%) | 70 (79.5%) | 68 (59.1%) | 0.005 |
| Number of antibiotic lines, mean±SD | 4.8±5.5 | 1.9±2.8 | <0.001 |
| Resistant bacteria, n (%) | 19 (21.6%) | 9 (7.8%) | 0.009 |
| Pseudomonas, n (%) | 18 (20.04%) | 8 (6.9%) | 0.008 |
Statistically significant p-values are presented in bold.
All-cause mortality was comparable between the two group (58% and 47%, p=0.16), as were mortality rates over time (Supplementary Fig. S3A). Age at the time of CLL diagnosis was significantly associated with mortality (p=0.002), while the presence of bronchiectasis was not. Additional predictors for mortality included Rai stages 2 or higher at diagnosis, CLL transformation, and requirements for unplanned hospitalizations (Supplementary Table S3 and Figs. S3–S4).
Two prior studies reported on subjects with HM among bronchiectasis cohorts; most CLL patients were heavily treated, especially with rituximab, many had undergone stem cell transplantation (SCT) and developed graft-vs-host disease, and most had acquired IgG deficiency.5,6 Our study, which began with a CLL cohort, allowed assessing the prevalence and risk factors for bronchiectasis among this population. None of the subjects in our study had undergone SCT, and 30% of the subjects with bronchiectasis were not treated for CLL at all. Despite this, a significant proportion, 43.3% of subjects had radiological evidence of bronchiectasis. This was associated with significant morbidity and thus represents clinically meaningful bronchiectasis.7
One prior study reported the prevalence of bronchiectasis among CLL patients which required immunoglobulin therapy for secondary antibody deficiency. Remarkably, the proportion of bronchiectasis was very similar to our study, 44%.8 In that study, 91% received treatment for CLL, including 78% who received anti-CD20 antibodies. We found that venetoclax treatment may be considered a risk factor for developing bronchiectasis in CLL patients, and a trend supporting rituximab treatment might also be a risk factor. Both of those treatments lead to severe depletion of B-lymphocytes and consequently impaired production of immunoglobulins. Laboratory evidence of immunodeficiency, such as low levels of IgG, IgA, and neutropenia, were also associated with bronchiectasis risk. Subjects with immunoglobulin deficiencies, whether acquired or primary (such as common variable immunodeficiency),9 commonly suffer from bronchiectasis. This further supports a cause-and-effect association. While rituximab therapy for HM5,8,10 or rheumatoid arthritis11,12 was associated with bronchiectasis in some publications, we are not aware of studies linking venetoclax and bronchiectasis.
Available CT scans were adequate for the diagnosis of bronchiectasis in over 97% (208/214 subjects), in-line with current data reporting high-sensitivity for diagnosing bronchiectasis in low-dose CT scans.13 Thus, the radiological diagnosis of bronchiectasis can frequently be accomplished from available imaging studies, performed for various indications.
We did not find an association between the presence of bronchiectasis and mortality.
In large series of subjects with CLL the three leading causes of death were the disease itself, non-CLL-related comorbidities, and other malignancies. Infections were the fourth most common cause of mortality.14,15 The association of mortality with Rai staging is well established,16 thus we believe that our cohort is a good representative of general subjects with CLL.
In addition to overt, patient-related clinical outcomes, such as respiratory infections and hospitalizations, bronchiectasis were also associated with isolation of resistant bacteria from respiratory tract cultures. We considered bacteria which are not part of the “core respiratory pathogens” in the Consensus Statement Regarding Initial Strategies to treat pneumonia in immunocompromised patients as resistant, since empiric therapy might not be adequate for their treatment.17 We believe this is clinically relevant: sputum cultures should be collected early, and wider spectrum antibiotics should be considered to treat respiratory infections in CLL patients and bronchiectasis or risk factors for it. On the other hand, detecting Pseudomonas or other resistant bacteria in cultures from subjects with CLL should raise suspicion for underlying bronchiectasis.
As a single-center, retrospective study with a modest sample size, our study has obvious limitations. Selection bias might result from including only subjects with available CTs, and since SCT is not available in our Medical Center. Despite those limitations, this is the largest study to assess CLL and bronchiectasis, and as such contributes important data.
In conclusion, bronchiectasis is common among patients with CLL, especially in the presence of specific risk factors, and results in increased morbidity.
Conflicts of InterestNone declared.