Bronchiectasis is a chronic respiratory disease characterized by a heterogeneous clinical syndrome of cough, sputum production and a history of pulmonary exacerbations.1 Despite an evident increase in prevalence and incidence of bronchiectasis during the last decade, licensed treatments are available nowadays for this disease neither in the United States of America nor in Europe. Several trials evaluating pharmacological treatments in bronchiectasis failed to reach their primary endpoints because of the wide heterogeneity of the disease in terms of aetiology, pathogenesis and clinical/radiological manifestations.2 For this reason, the bronchiectasis scientific community has recently focused its efforts on the identification of treatable traits of the disease, as clinical features describing differences among patients (namely, phenotypes) and underlying biological mechanisms explaining specific pathological processes (namely, endotypes).3
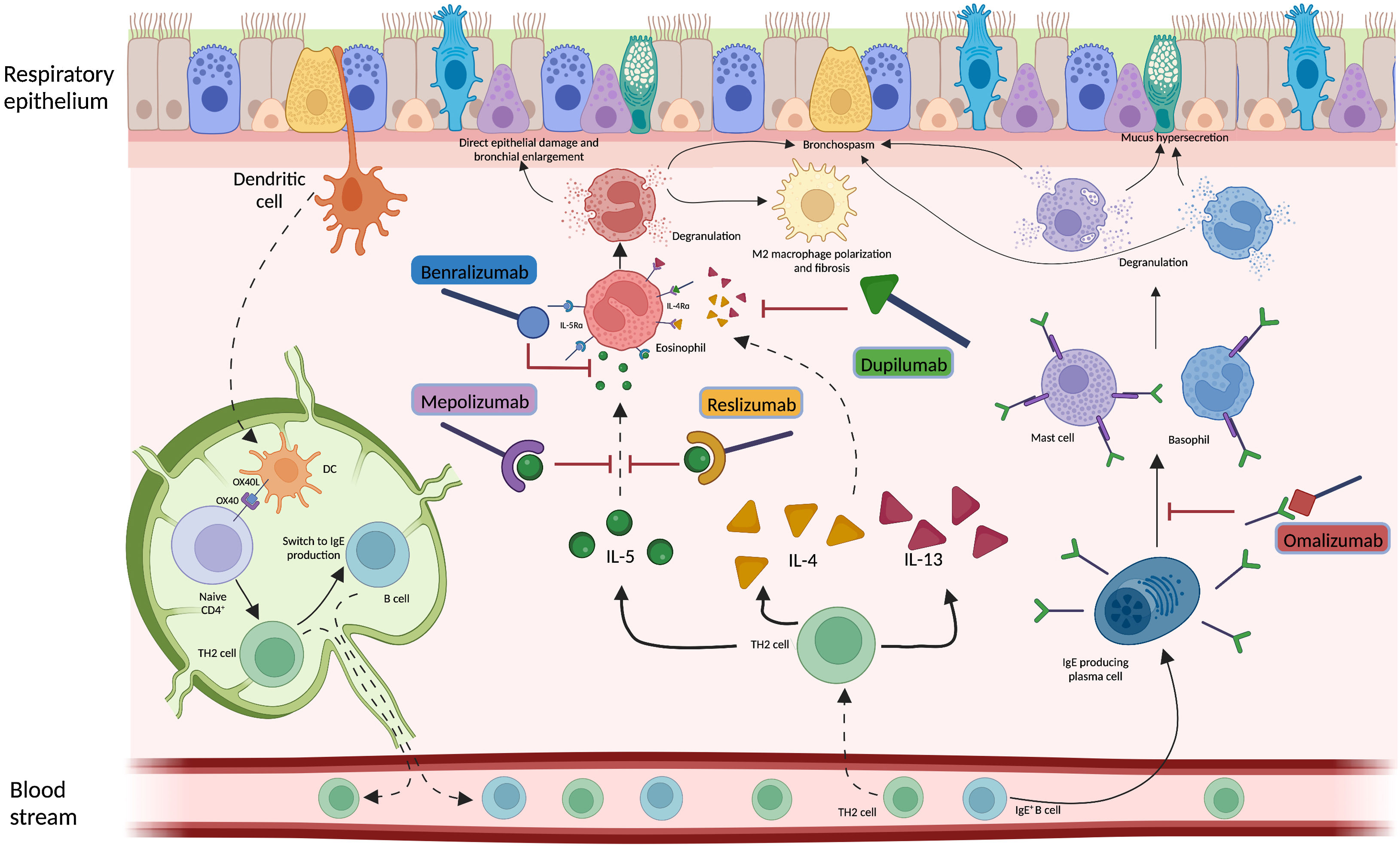
Airway inflammation is one of the main components of the vicious vortex in bronchiectasis that promotes and perpetuates the pathogenesis of the disease.4 In most of the cases, the airway inflammatory response is driven by neutrophils with a dysregulated production of neutrophil extracellular traps (NETs) and release of proteases.5 However, up to 25–30% of bronchiectasis patients might have an eosinophilic inflammation.6,7 International researchers recently highlighted a correlation between serum and sputum eosinophils in bronchiectasis similarly to what is already known in COPD and asthma.7 Oriano and colleagues recently identified a T2-high endotype in bronchiectasis patients (regardless of the presence of co-existing asthma) through either raised serum eosinophils or increased oral fractional exhaled nitric oxide (FeNO). This population is characterized by an increased disease severity, a worse pulmonary function and a higher burden on quality of life. Notably, up to 5% of bronchiectasis patients included in this study showed both a T2-high endotype and a status of “frequent exacerbator” despite optimized medical therapy and respiratory physiotherapy.6 Moreover, serum eosinophils have a prognostic impact in bronchiectasis, as patients with a raised eosinophilic count have a shortened time to exacerbations compared to patients with low eosinophils.7 Finally, some authors also speculated about a possible role of blood eosinophils in guiding the use of inhaled corticosteroids in bronchiectasis.8,9
Considering the hypothesis that eosinophilic inflammation could be the actual driver of bronchiectasis pathogenesis and not just a co-occurrent factor, targeting this pathway could modify the natural history of the disease in a specific subgroup of patients. Several biological anti-inflammatory drugs are available on the market for other conditions, such as allergic bronchopulmonary aspergillosis (ABPA) and asthma, aiming at controlling T2 inflammation through this precision medicine approach. Treatments targeting a T2-high endotype in people with both bronchiectasis and severe asthma have already shown some benefits, although in limited case series.6,10,11 As an example, five patients with clinically and radiologically significant bronchiectasis from the previously cited paper by Oriano et al. were treated with biological drugs directed against either soluble IL-5 or IL-5 receptor due to coexistent eosinophilic asthma with a reduction in exacerbations and prescription of oral corticosteroids after 6, 12 and 24 months of treatment.6 These results are consistent with other findings available in literature. A different Italian cohort of four patients with bronchiectasis and eosinophilic asthma treated with mepolizumab showed a significant improvement in the Asthma Control Test questionnaire, a reduction in exacerbations, an increase in FEV1 and a decrease in both sputum and serum eosinophils after one year of treatment.10 Similar results have been also demonstrated in a German cohort of 21 patients with eosinophilic endotype bronchiectasis treated with either mepolizumab or benralizumab who showed a functional and quality of life improvement and a decrease in eosinophils count and sputum volume after six months of therapy.11 Even considering the low sample size of these studies, these findings suggest that IL-5 could be key in the pathogenesis and progression of the disease in some bronchiectasis patients and concur to identify eosinophilic inflammation as a treatable trait in bronchiectasis.
However, different questions remain unresolved. First, asthma, ABPA and other pulmonary or extra-pulmonary conditions are often associated with eosinophilic inflammation and the use of T2-directed biological drugs in this setting might improve clinical outcomes regardless of the presence or extension of bronchiectasis.12 Whether the overall clinical improvement shown in the previously cited studies should be attributed to the drug effect on bronchiectasis pathophysiology is difficult to prove. Moreover, data on safety and efficacy of biological drugs in clinically and radiologically significant bronchiectasis with a T2-high inflammation and with neither severe asthma nor ABPA are urgently needed, while the identification of this peculiar population might be questionable. Asthma and bronchiectasis are often partners in a complex relationship.13 Future pragmatic RCTs evaluating biological drugs in bronchiectasis patients with a T2-high inflammation should consider excluding patients with severe asthma and/or ABPA, but they should include those with mild/moderate asthma patients to guarantee a good generalizability of the findings. Second, the relationship between neutrophilic and eosinophilic inflammation, that are often considered mutually exclusive in COPD and asthma, should be further investigated in bronchiectasis. The possible coexistence of these two inflammatory patterns represents a challenge in the management of bronchiectasis, as the effects of biological drugs in these individuals are unknown. Third, longitudinal changes of airway inflammation through time might be expected in bronchiectasis patients and shifts from eosinophilic to neutrophilic or mixed inflammation (and vice versa) could happen. Forth, the relationship between inflammation and microbiome is under evaluation in bronchiectasis, with a diverse prevalence of several microbial species in patients with low eosinophils when compared to those with high eosinophils. However, this relationship should be better elucidated, especially for pathogens associated with high burden of disease. Pseudomonas aeruginosa, for instance, has shown a greater dominance in patients with low eosinophils, while its relative abundance has been related to T2-high patients.7 Further research should be focused on the evaluation of this association to define to which extent eosinophilic inflammation could be linked to specific pathogens and how biological drugs could affect the host response against them. Fifth, the role for biologics directed against T2 inflammation markers different from IL-5 (summed up in Fig. 1) should be also investigated in bronchiectasis patients.
Biological drugs represent an overall huge opportunity for bronchiectasis patients with an underlying T2-high inflammatory endotype. Larger, multicentric trials are needed and expected in the upcoming years to provide an answer to the previously listed unresolved questions and to give clinicians new drugs to improve outcomes of bronchiectasis patients. The precision medicine era has finally begun, and exciting times are ahead for patients, clinicians and researchers dealing with bronchiectasis.
Conflict of InterestMattia Nigro, Edoardo Simonetta and Miguel Ángel Martínez-García have nothing relevant to declare concerning the content of this editorial. Stefano Aliberti has received fees from INSMED, ZAMBON, Astrazeneca, CSL Behring GmbH, Grifols, Fondazione Charta, Boehringer Ingelheim, CHIESI, ZCUBE Srl, MENARINI, MSD Italia S.r.l., royalties from McGRAW HILL, grants from INSMED, CHIESI and Fisher & Paykel, payments by GlaxoSmithKline Spa and has participated on boards organized by INSMED, AstraZeneca and MSD Italia S.r.l.