Chronic obstructive pulmonary disease (COPD) is strongly associated with the development of community-acquired pneumonia (CAP). Limited data are available on risk factors for difficult to manage bacteria such as Pseudomonas aeruginosa in COPD patients with CAP. Our objective was to assess the microbiological patterns associated with risk factors that determine empiric antibiotic therapy in hospitalized COPD patients with CAP.
MethodsWe performed a secondary data analysis of an international, multicenter, observational, point-prevalence study involving hospitalized COPD patients with CAP from March to June 2015. After identifying the risk factors associated with different microorganisms, we developed a scoring system to guide decision-making about empiric anti-pseudomonal antibiotic therapy in this population.
ResultsWe enrolled 689 hospitalized COPD patients with CAP with documented microbiological testing. The most frequent microorganisms isolated were Streptococcus pneumoniae (8%) and Gram-negative bacteria (8%), P. aeruginosa (7%) and Haemophilus influenzae (3%). We developed a scoring system incorporating the variables independently associated with P. aeruginosa that include a previous P. aeruginosa isolation or infection (OR 14.2 [95%CI 5.7–35.2]), hospitalization in the past 12 months (OR 3.7 [1.5–9.2]), and bronchiectasis (OR 3.2 [1.4–7.2]). Empiric anti-pseudomonal antibiotics were overutilized in COPD patients with CAP. The new scoring system has the potential to reduce empiric anti-pseudomonal antibiotic use from 54.1% to 6.2%.
ConclusionsCOPD patients with CAP present different microbiological profiles associated with unique risk factors. Anti-pseudomonal treatment is a critical decision when selecting empiric antibiotic therapy. We developed a COPD scoring system to guide decision-making about empiric anti-pseudomonal antibiotic therapy.
Chronic obstructive pulmonary disease (COPD) currently affects more than 250 million people worldwide, and by the year 2060, it is estimated that COPD will account for more than 6 million deaths annually, making it the fifth leading cause of death.1 Community acquired pneumonia (CAP) and COPD exacerbations are two important complications associated with increased morbidity, mortality, costs, hospitalizations, and readmissions.2–7 Furthermore, CAP in COPD patients compared with COPD exacerbations is associated with longer length of hospital stay, higher mortality and greater economic impact.8 COPD is a heterogeneous disease in which multiple risk factors may lead to the development of CAP.9,10 When CAP occurs in COPD patients, clinicians are challenged in deciding the appropriate selection of antibiotics.
Recently published CAP clinical practice guidelines recommend microbiological confirmation and treatment for Pseudomonas aeruginosa in patients with prior respiratory infection or colonization with P. aeruginosa, recent hospitalization, exposure to parenteral antibiotics, and locally validated risk factors.11 Previous studies have reported that hospitalized CAP patients with COPD have more infections caused by P. aeruginosa, especially those with bronchiectasis and severe disease (Forced expiratory volume in one second [FEV1<50%]).12–14 However, most of these studies recruited small sample sizes, were conducted in single centers, evaluated a unique pathogen, or considered COPD as a homogeneous entity.12–16 Limited data are available on the association of risk factors in COPD patients with CAP and difficult-to-manage bacteria, such as P. aeruginosa, other Gram-negative bacteria (GNB), and Methicillin Resistant Staphylococcus aureus (MRSA). The aim of this study was to assess the microbiological patterns associated with risk factors that determine empiric antibiotic therapy in hospitalized COPD patients with CAP.
MethodsStudy designThis study was designed using data retrieved from an international multicenter, observational, point-prevalence study (Global Initiative for Methicillin-resistant Staphylococcus aureus Pneumonia [GLIMP]).17 Adult immunocompetent COPD patients hospitalized with CAP from 37 countries in all continents were selected.17
The patients were enrolled on four randomly selected days. We included patients with bacterial tests done (blood and respiratory cultures, pneumococcus and legionella urinary antigen and/or influenza testing) during the first 24h of admission. Respiratory samples collection included sputum, pleural fluid, endotracheal aspirate, and/or bronchoalveolar lavage according to local standard protocols. Investigators did not actively participate or alter the clinical decisions, procedures, management of microbiological samples, or treatment decisions, which were performed according to local protocols and local standards of care.
Inclusion and exclusion criteriaIn the original cohort of GLIMP, all adult patients (>18 years old) admitted to the hospital with CAP were screened for the study inclusion. Patients with a diagnosis of hospital-acquired or ventilator-associated pneumonia or tracheotomized were excluded.
COPD group stratificationData collection were stratified according to the following COPD groups of potential risk factors found in the literature: demographics (age, gender)18; prior pulmonary disease (asthma, bronchiectasis, and FEV1)12,13; non-pulmonary comorbidities (diabetes, heart failure, liver disease, obesity, stroke)19; chronic medication use (inhaled or systemic corticosteroids and oxygen therapy)12,13,20 and healthcare system or pathogen exposure during the previous year (previous hospitalizations in the past 12 months, intravenous antibiotic use in the past 12 months, nursing home residence, and P. aeruginosa, MRSA or extended spectrum beta-lactamase bacteria exposure).13,15
DefinitionsCAP was defined by the presence of pulmonary infiltrates diagnosed by chest imaging (chest radiography, lung ultrasound or computed tomography [CT]) during the first 48h of hospital admission and at least one of the following: (a) cough with or without sputum production; (b) fever (rectal or oral temperature >37.8°C) or hypothermia (<36°C) and/or (c) systemic inflammation (leukocytosis>10,000cm−3, leucopenia<4000cm−3, bandemia>10%, increased C-reactive protein or procalcitonin levels).
COPD was defined by Global initiative for Obstructive Lung Disease (GOLD) criteria,21 as patient>40 years old who has dyspnea, chronic cough or sputum production, and/or a history of exposure to risk factors for the disease, with a post-bronchodilator FEV1/FVC ratio under 0.7.
Bronchiectasis was defined as the presence of abnormal dilatation of bronchi on CT scan of the thorax as reported in the case report form.
Microbiology pathogens were stratified in the following groups: (a) None (no pathogen isolated), (b) P. aeruginosa, (c) GNB, (d) Haemophilus influenzae, (e) S. aureus, (f) Streptococcus pneumoniae, (g) Streptococcus spp., (h) Mixed flora and (i) Atypical bacteria. The GNB group included Acinetobacter baumannii, Coxiella burnetii, Escherichia coli, Klebsiella pneumoniae, Proteus spp., Moraxella catarrhalis, Enterobacter spp., Pasteurella multocida, Pseudomonas pseudomallei, Salmonella spp., and Serratia spp. Atypical bacteria included Chlamydia pneumophila, Chlamydia psittaci, Legionella spp., and Mycoplasma pneumoniae. Viruses, fungi and mycobacteria, since they are not treated with conventional antibiotics were included in the “non-bacterial group”.
Treatment groups. Anti-pseudomonal antibiotic therapy was stratified in five groups: (1) anti-pseudomonal beta-lactam monotherapy (piperacillin-tazobactam, imipenem, meropenem, cefepime, ceftazidime, aztreonam); (2) fluoroquinolone monotherapy (ciprofloxacin or levofloxacin); (3) combination of anti-pseudomonal (beta-lactam plus either aminoglycoside, fluoroquinolone or colistin or fluoroquinolone plus aminoglycoside, respectively); (4) non-conventional antipseudomonal antibiotics (monotherapy with colistin or aminoglycoside [gentamicin, tobramycin, or amikacin], or combination of colistin plus fluoroquinolone, or colistin plus aminoglycoside; and (5) patients without anti-pseudomonal treatment.
Anti-pseudomonal antibiotic use was classified as correct use (patients with CAP caused by P. aeruginosa and treated with anti-pseudomonal agents); correct non-use (patients without P. aeruginosa and not empirically treated for P. aeruginosa CAP); undertreatment (patients with CAP caused by P. aeruginosa and not treated for P. aeruginosa CAP); and overtreatment (patients without CAP caused by P. aeruginosa that received treatment with anti-pseudomonal antibiotics).
Data collectionResearch Electronic Data Capture (REDCap) was used to collect and manage all data.22 Data collection included demographics, respiratory and non-respiratory comorbidities, chronic therapies, other non-medical conditions, severity of pneumonia, empiric antibiotic usage, and microbiological tests results.
Ethical considerationsThis study was approved by the Institutional Review Board of the University of Texas Health San Antonio, (coordinating center located in San Antonio, Texas, USA with number HSC20150184E). Full data were confidential according to the current legislation. Patient identifiers were removed before analysis to maintain strict patient confidentiality. For these reasons, the institutional review board decided informed consent was not necessary. All associated centers followed local, regional, or national ethics regulations. The study was designed and conducted in accordance with the Declaration of Helsinki.
Statistical analysisThe sample size was defined by the total number of immunocompetent COPD patients with CAP on the dates selected by the investigators in GLIMP study.17 Two-sided Chi-square or Fisher Exact tests were used for the analysis of categorical variables, when appropriate. The normal distribution of continuous variables was tested using the Kolmogorov–Smirnov test. The Student's t-test was used to compare continuous variables expressed as means and standard deviation (SD) in case of a parametric distribution. Risk factors associated with CAP pathogen (P. aeruginosa, GNB, MRSA, and S. pneumoniae) were assessed with a logistic regression analysis. Multivariate analysis was performed in all the COPD patients with microbial tests performed for CAP pathogens, and a scoring system (PAS-COPD) based on rounded β value was developed for P. aeruginosa with independent risk factors. The discriminative ability of the PAS-COPD to predict the presence of PA was assessed by the area under the receiver operating characteristic (ROC) curve. The ROC curves were used to identify the optimal cutoff values for the outcome associations. All statistical analyses were performed using SPSS 24.0 (SPSS Inc., Chicago, IL, USA), and a p-value<0.05 was considered statistically significant.
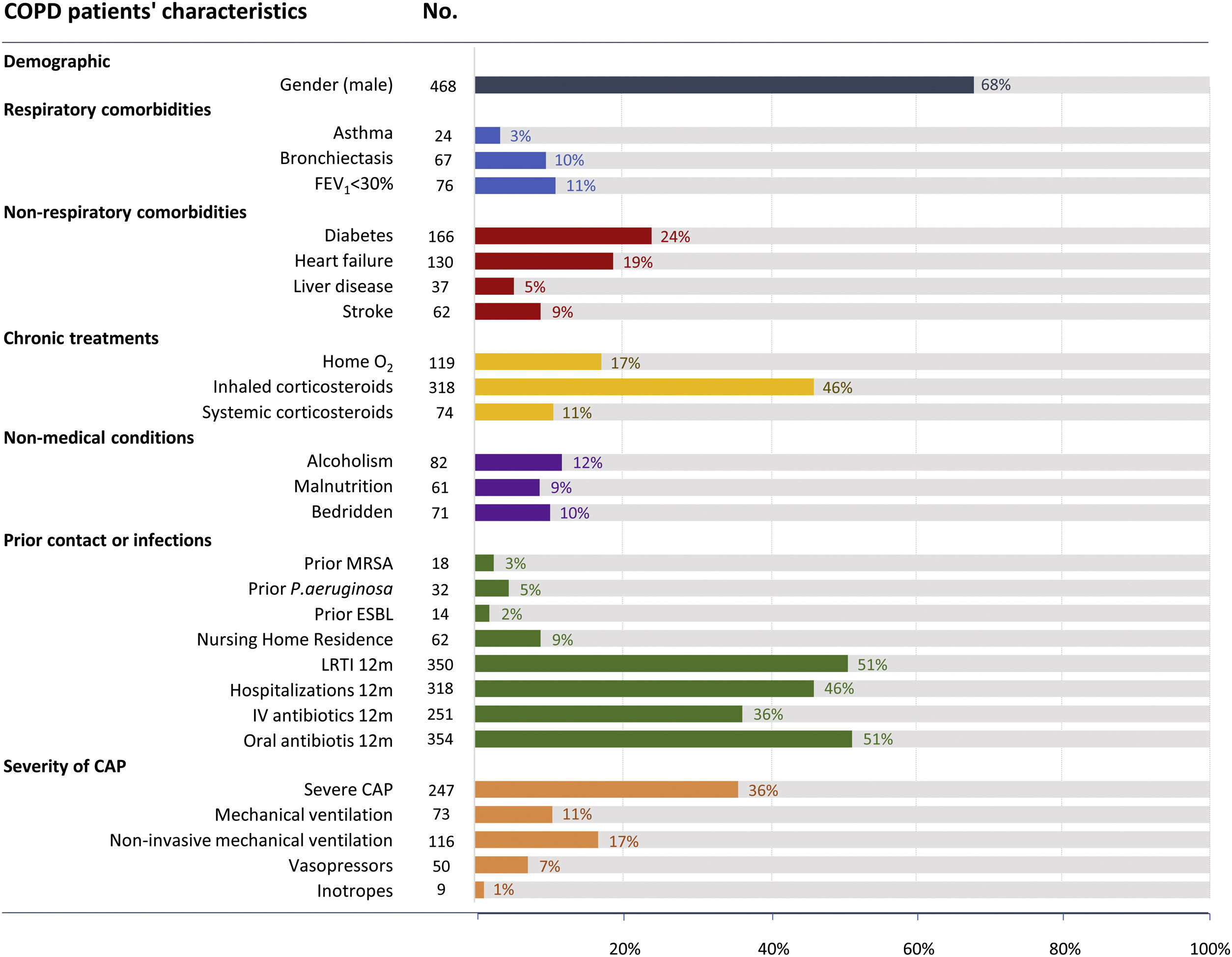
ResultsAmong 3217 hospitalized patients with CAP and microbiological testing performed, 689 (21%) patients were immunocompetent with COPD. COPD patients had a mean age of 72 (±11) years, 67% were male, 11% had very severe airflow limitation (FEV1≤30%), 10% had bronchiectasis, and 5% had prior P. aeruginosa infection (Fig. 1). The most frequently identified demographic characteristic in addition to being male was the prior use of oral antibiotics in the past 12 months (n=354 [51%]), low respiratory tract infections in the past 12 months (n=350 [51%]), hospitalization in the past 12 months (n=318 [46%]) and use of inhaled corticosteroids (n=318 [46%]), respectively (Fig. 1).
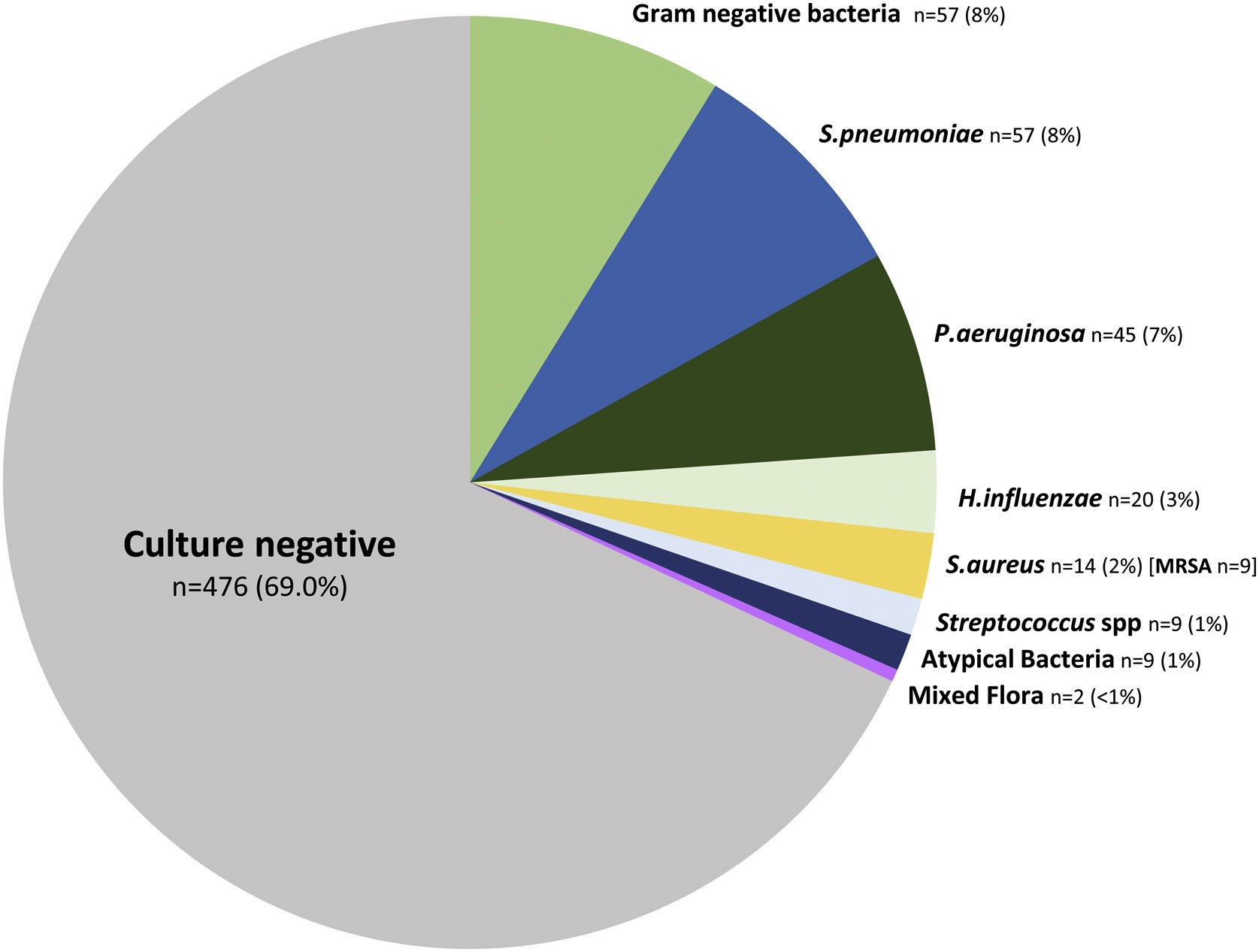
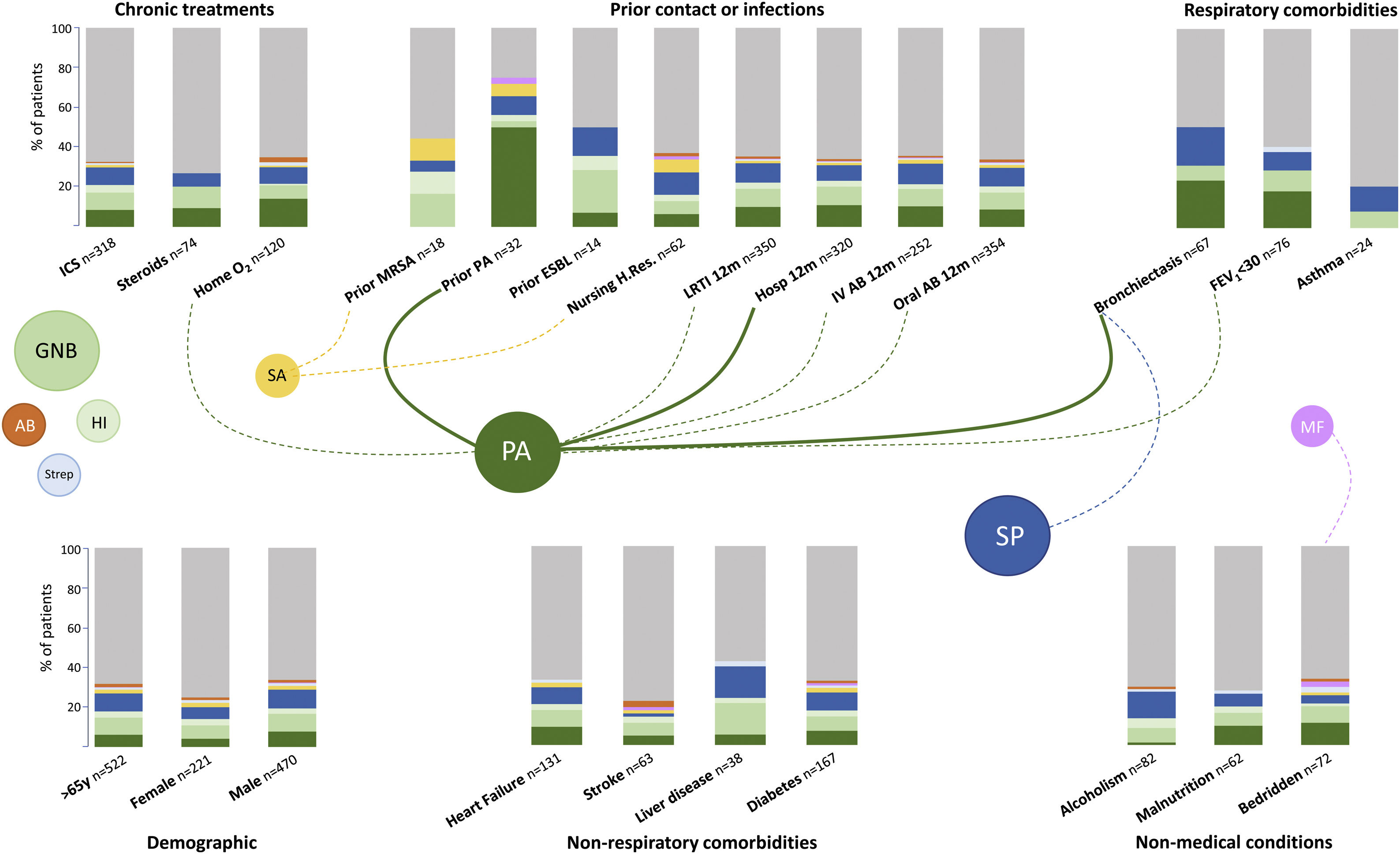
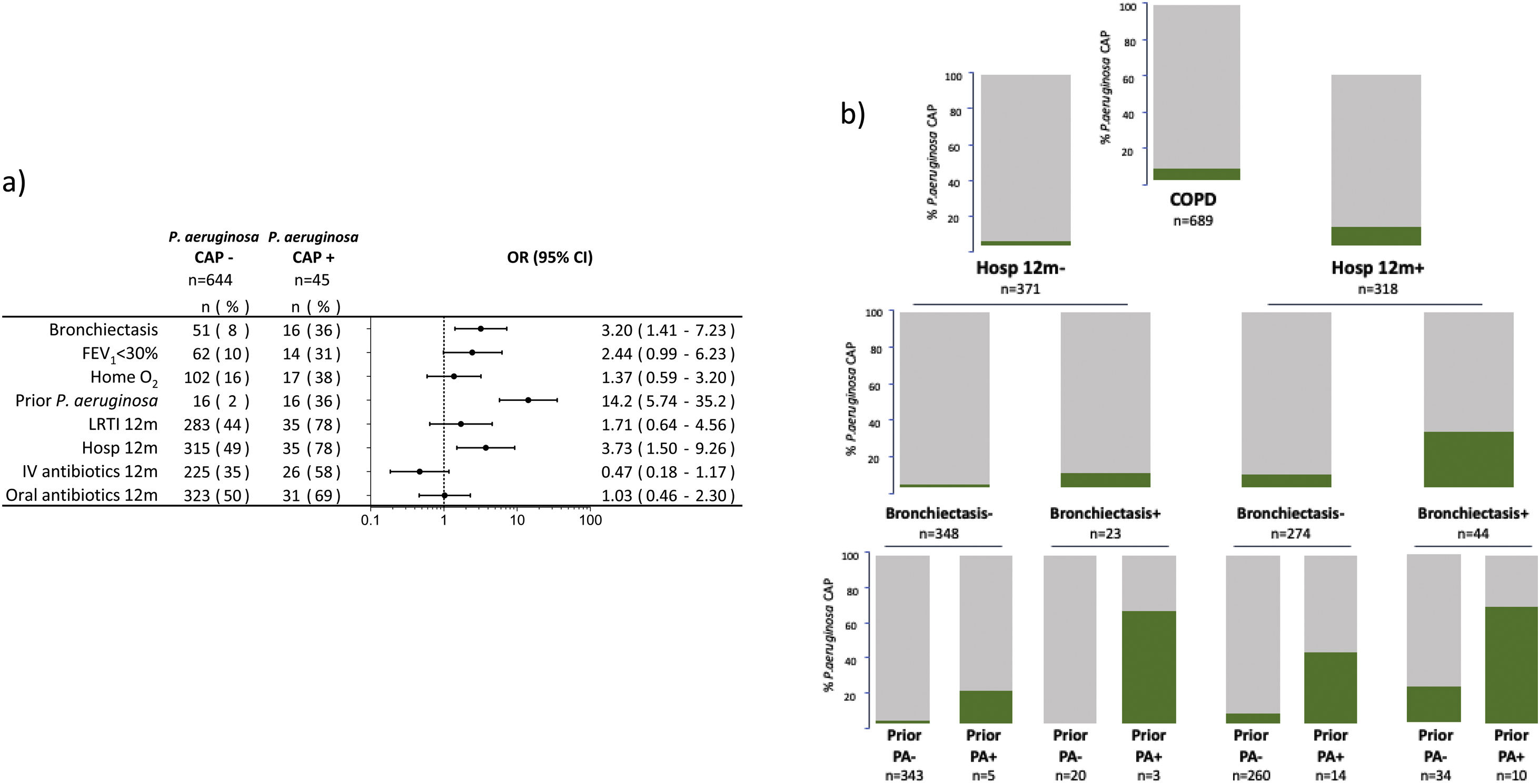
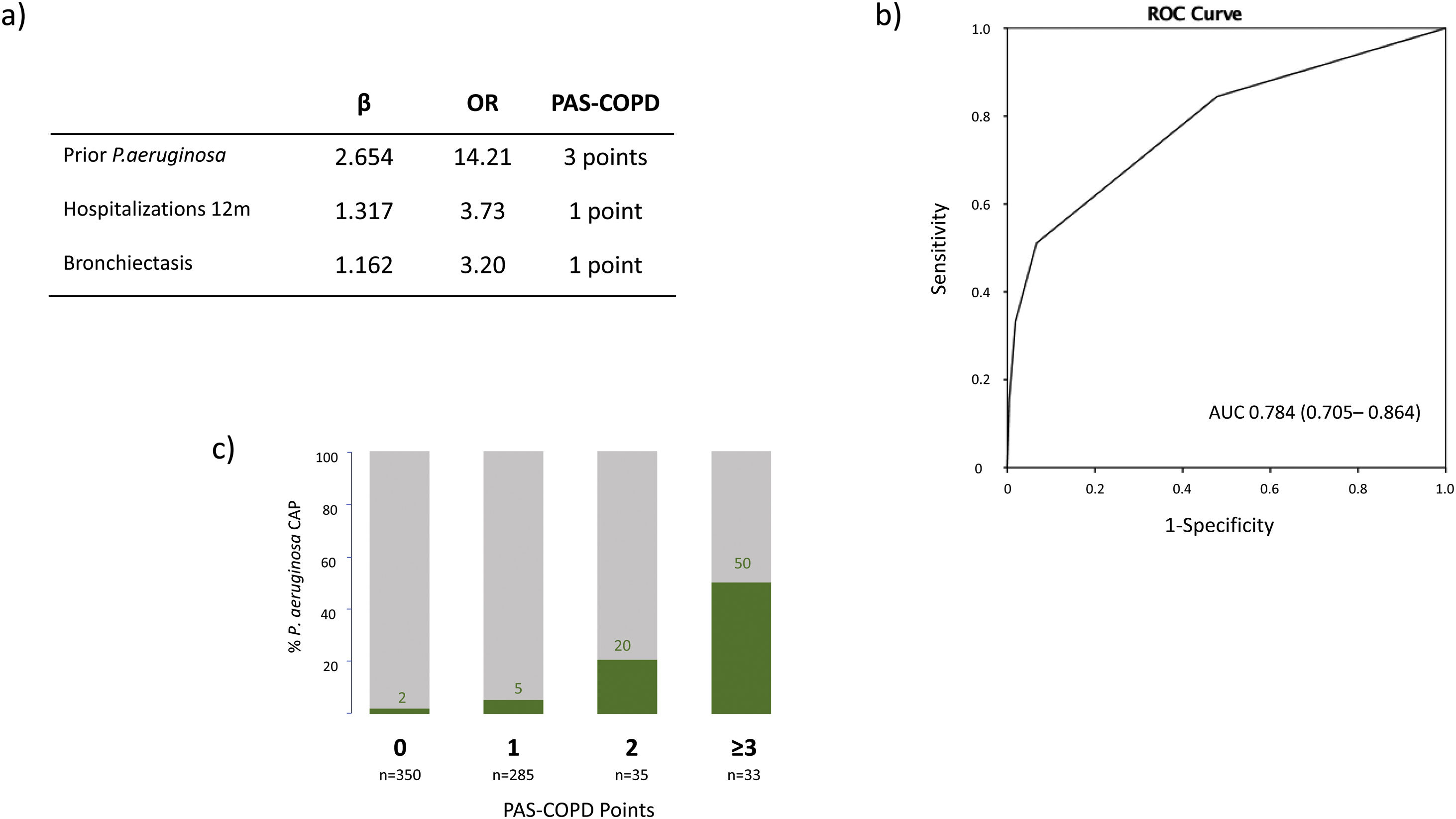
The microbiological identification occurred in 213 (31%) of all the COPD patients with CAP (Fig. 2). The most prevalent pathogens were GNB (n=57; 8%), S. pneumoniae (n=57; 8%), P. aeruginosa (n=45; 7%), H. influenzae (n=20; 3%), and S. aureus (n=14; 2%, MRSA n=9; 1.9%), respectively (Fig. 2). Distinct microbiological patterns for P. aeruginosa, S. pneumoniae, S. aureus and mixed anaerobic flora were associated with risk factors in COPD patients (Fig. 3). P. aeruginosa was independently associated with a previous P. aeruginosa isolation or infection (OR 14.2 [95%CI 5.7–35.2]), hospitalization in the past 12 months (OR 3.7 [1.5–9.2]), and bronchiectasis (OR 3.2 [1.4–7.2]) (Figs. 3 and 4a). In the bivariate analysis other pathogens such as S. pneumoniae was associated with bronchiectasis in COPD patients with CAP (19% vs. 7%; p-value<0.01), and S. aureus was associated with prior MRSA isolation (11% vs. 2%; p-value<0.049) and nursing home residence (7% vs 2%; p-value<0.03), but none of these conditions were independently associated with S. aureus CAP in the multivariate analysis (Fig. 3). Mixed anaerobic flora was associated with being bedridden (2% vs 0%, p-value=0.01) (Fig. 3). Inhaled and oral corticosteroids treatment was not related to any microorganism. We developed a decision tree analysis focused on assessing the risk of P. aeruginosa CAP in three independently associated COPD risk factors (hospitalization in the past 12 months, bronchiectasis, and prior P. aeruginosa infection/colonization) (Fig. 4b). For instance, patients with bronchiectasis and without any previous hospitalizations in the past 12 months, and no evidence of prior P. aeruginosa, had no P. aeruginosa CAP (0%), whereas 70% of patients with previous hospitalizations, bronchiectasis, and prior P. aeruginosa infection/colonization had P. aeruginosa CAP. A P. aeruginosa CAP score in COPD (PAS-COPD) was created to define the prevalence of P. aeruginosa with 3 points for prior P. aeruginosa infection/colonization, 1 point for hospitalization in the past 12 months, and 1 for bronchiectasis, for a total score of 5 (Fig. 5a). The ability of PAS-COPD to identify patients with P. aeruginosa was an area under the curve–ROC of 0.784 (0.705–0.864, p<0.001) (Fig. 5b). The PAS-COPD was stratified as 0, 1, 2, or ≥3 points with a P. aeruginosa CAP prevalence of 2%, 5%, 20% and 50%, respectively (Fig. 5c).
Microbiological patterns associated with distinct COPD risk factors in hospitalized patients with CAP. Bar colors represent the different percentages of bacterial isolations for each risk factor. Dashed lines represent a statistically significant difference (prevalence of the bacteria comparing patients with and without the specific risk factor) in the bivariate and the solid lines in the multivariate analysis, respectively. Footnote Fig. 3. GNB: Gram negative bacteria, SP: S. pneumoniae, PA: P. aeruginosa, SA: S. aureus, HI: H. influenzae, Strep: Streptococcus spp, AB: Atypical bacteria, MF: Mixed flora. ICS: inhaled corticosteroids, MRSA: methicillin resistant Staphylococcus aureus, ESBL: extended spectrum β-Lactamases, LRTI: low respiratory tract infection, IV: intravenous, Ab: antibiotic, Hosp: hospitalization, Nursing H.Res: nursing home residence, FEV1: forced expiratory capacity in one second.
(a) Multivariate analysis of the risk factors associated with P. aeruginosa CAP among hospitalized patients with COPD. (b) Bar graphs decision tree analysis representing the prevalence of P. aeruginosa CAP by the three independently associated risk factors (prior hospitalization in the past 12 months, bronchiectasis, and prior P. aeruginosa infection or colonization). Footnote Fig. 4. Hosp: hospitalization, PA: P. aeruginosa, CAP: Community acquired pneumonia.
(a) Table representing the points assigned to the three independently associated risk factors included in the Pseudomonas aeruginosa score (PAS) for COPD patients. (b) Receiver operating curve of the PAS-COPD performance to predict P. aeruginosa CAP. (c) Actual distribution of the prevalence of P. aeruginosa CAP according to the number of points of the PAS-COPD.
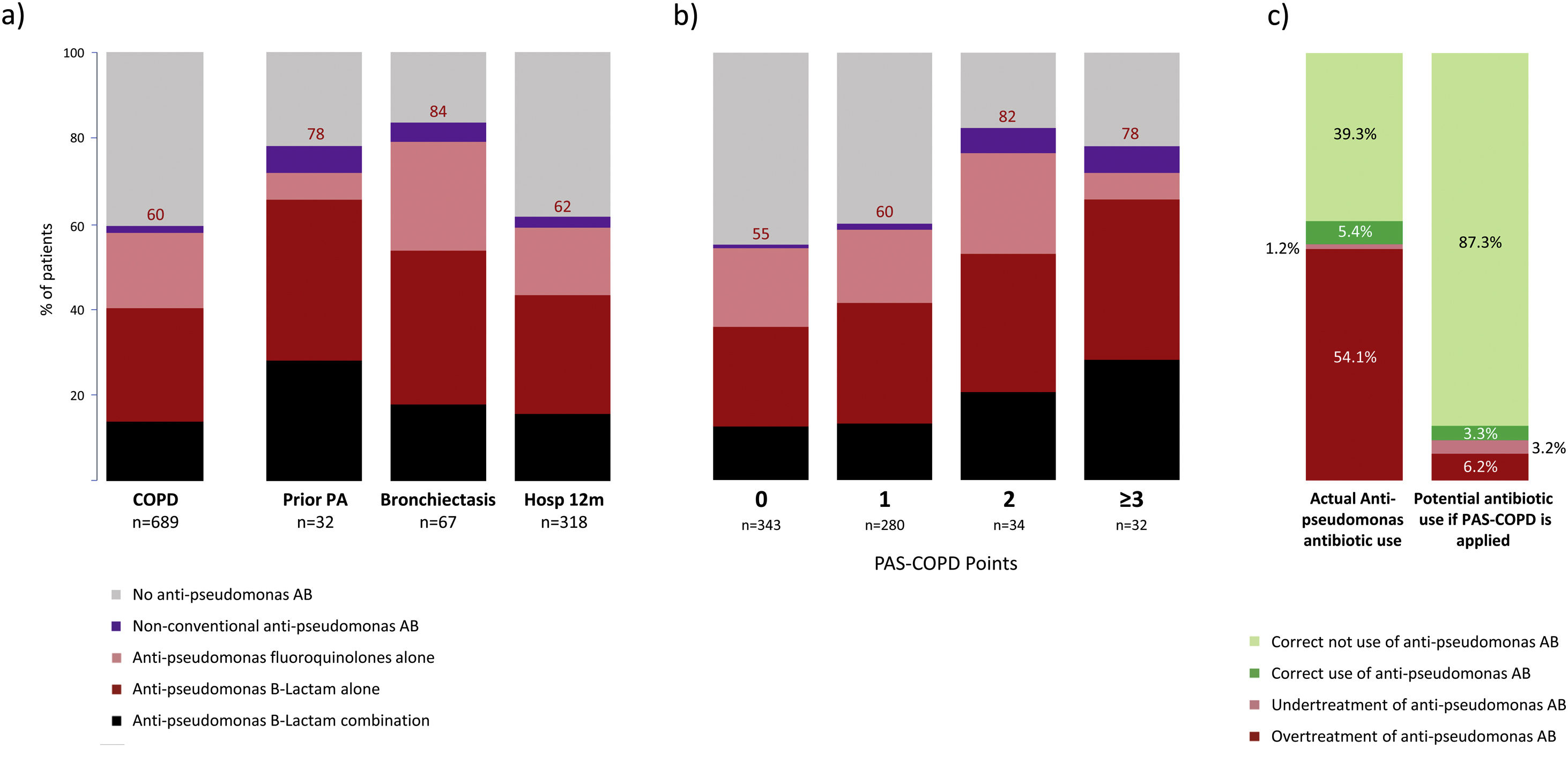
Empiric anti-pseudomonal antibiotics were used in 413 (60%) of all COPD patients hospitalized with CAP, with 197 (62%) for patients with prior hospitalization in the past 12 months, 25 (78%) for those with a prior P. aeruginosa infection/colonization, and 56 (84%) for those with bronchiectasis (Fig. 6a). The most frequently prescribed antibiotics were anti-pseudomonal beta-lactam monotherapy (n=179; 26%), anti-pseudomonal fluoroquinolones (n=124; 18%), and combination therapy (n=96; 14%) of a beta-lactam with either a fluoroquinolone, aminoglycoside, or colistin, respectively. A distinct treatment pattern was not found of anti-pseudomonal antibiotic use according to the above-mentioned risk factors (Fig. 6a). Patients with a PAS-COPD of 0, 1, 2, and ≥3 received anti pseudomonal treatment in 55%, 60%, 82%, and 78% of the cases, respectively (Fig. 6b). The prevalence of appropriate use, appropriate non-use, undertreatment, and overtreatment of anti-pseudomonal antibiotics were 5.4%, 39.3%, 1.2%, and 54.1%. Avoiding antipseudomonal agents in patients with PAS-COPD score≤1 (P. aeruginosa CAP prevalence≤5%) the potential appropriate use, appropriate non-use, undertreatment, and overtreatment of anti-pseudomonal antibiotics would be 3.3%, 87.2%, 3.2%, and 6.2%, respectively (Fig. 6c).
(a) Actual empiric anti-pseudomonal antibiotic use for COPD patients stratified according to the three independent risk factors associated to P. aeruginosa CAP. (b) Actual empiric anti-pseudomonal antibiotic use for COPD patients stratified according to PAS-COPD score. (c) Distribution of appropriate use, appropriate non-use, undertreatment and overtreatment of empiric anti-pseudomonal antibiotics in the actual cohort of COPD patients with CAP and the potential empiric anti-pseudomonal antibiotic utilization if the PAS-COPD were applied in clinical practice. Footnote Fig. 6. Hosp: hospitalization, PA: P. aeruginosa; PAS-COPD: P. aeruginosa score for COPD.
Distinct microbiological patterns for P. aeruginosa and S. pneumoniae were associated with specific risk factors in COPD patients. P. aeruginosa CAP was associated with previous infection/colonization with P. aeruginosa, hospitalization during the previous 12 months, and bronchiectasis. Anti-pseudomonal antibiotics are routinely overused in COPD patients with CAP. A PAS-COPD score might help to rationalize the use of anti-pseudomonal agents in COPD patients with CAP.
In the present study, P. aeruginosa was the predominant microorganism associated with a higher number of risk factors in hospitalized COPD patients with CAP. P. aeruginosa CAP was independently associated with prior P. aeruginosa infection/colonization, prior hospitalization in the past 12 months, and bronchiectasis. These results are aligned but somewhat divergent from prior evidence suggesting that COPD risk factors are heterogeneous.12–14,18,20,23 Similar risk factors have been described in hospitalized patients with CAP or in patients with COPD exacerbations.24–28 Prior infection/colonization with P. aeruginosa suggests that this pathogen may remain in the airways of COPD patients until a point of disbalance causing pneumonia. In addition, previous hospitalizations in the past 12 months suggests that a previous contact with the healthcare system may increase the risk of acquiring a pathogen related to previous contamination or exposure. Chronic bacterial infection is prevalent in patients with bronchiectasis and COPD and are usually difficult to eradicated despite multiple antibiotic treatments.29–32 Our data support the association of a prior P. aeruginosa infection/colonization, prior hospitalization in the past 12 months, and bronchiectasis with P. aeruginosa CAP in COPD patients. Poor pulmonary function testing, although previously associated with P. aeruginosa, seems to be linked to other overlapping conditions, such as prior infection/colonization with P. aeruginosa or the presence of bronchiectasis. Therefore, our stratification was limited by identifying patients with a diagnosis of very severe COPD according to the FEV1 ratio below 30%. Similar findings have been suggested in patients with acute exacerbations of COPD or CAP.26,33–35
One group of pathogens, namely GNB, was not independently associated with unique COPD risk factors. S. pneumoniae, which was the most prevalent microorganism, was found to be associated in the univariate analysis, but not in the multivariate analysis in COPD patients with bronchiectasis that developed CAP. As mentioned above, bronchiectasis in patients with COPD was previously associated with increased bronchial inflammation, frequent airway colonization by several microorganisms, and severe airflow obstruction.36 Finally, low prevalent pathogens, such as H. influenzae (3%) and S. aureus (2%), were not associated with COPD risk factors. Our results support the recommendations of the 2019 American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) clinical practice guidelines for the management of CAP, where MRSA and P. aeruginosa should be detected and treated in patients with pathogen specific risk factors.11 In patients with COPD requiring hospitalization for CAP, the most important pathogen for which the conventional treatment should be altered was P. aeruginosa. Future studies should explore how these risk factors interact and how they can increase the risk of P. aeruginosa infection.
A P. aeruginosa decision tree and a newly developed P. aeruginosa score suggest that patients with COPD and CAP have a different prevalence of P. aeruginosa infection according to presence or absence of the combination risk factors. Previously designed scores attempting to identify multidrug resistant bacteria, such as P. aeruginosa, considered COPD a risk factor with varying results.15,37,38 However, these scores were not specific for P. aeruginosa and did not focus on patients with COPD, but rather used COPD as an individual risk factor. We developed a simple score with three variables independently associated with P. aeruginosa among patients with COPD and hospitalized with CAP. Our PAS-COPD can be dichotomized to separate a low (<6%) vs. high (20% or higher) prevalence P. aeruginosa CAP group that may potentially guide rational selection of appropriate anti-pseudomonal antibiotics. A low PAS-COPD score (0 or 1 point) suggests that patients may receive CAP antibiotics without any anti-pseudomonal coverage; in contrast a high PAS-COPD score suggests the need of anti-pseudomonal coverage. This finding is significant because it suggests that only 10% of COPD patients hospitalized with CAP may need anti-pseudomonal coverage.
The largest motivation to perform this study was the clinical concern of excessive anti-pseudomonal antibiotic usage among COPD patients that developed CAP. Our clinical gestalt was consistent with what we found in the actual anti-pseudomonal antibiotic utilization. Our results suggest that two-thirds of COPD patients that develop CAP received at least one anti-pseudomonal antibiotic. Although empirical antibiotic therapy covering P. aeruginosa is indicated in certain patients,39 the high anti-pseudomonal antibiotic utilization (>60%) is not justified given the low prevalence of P. aeruginosa CAP (7%). Other authors have suggested that anti-pseudomonal antibiotics are overused in patients with CAP, particularly in those with COPD.37 It is interesting that the most commonly used anti-pseudomonal antibiotics were beta-lactam antibiotic monotherapy (27%), fluoroquinolones monotherapy (17%), followed by combinations of a beta-lactam antibiotic plus a fluoroquinolone, colistin, or aminoglycoside (14%). There is great concern about antimicrobial resistance, and this leads toward higher antimicrobial usage and in particular combination of anti-pseudomonal antibiotics. Recent CAP guidelines recommend using anti-pseudomonal agents in patients with prior isolation of this organism, especially from the respiratory tract; recent hospitalization; and exposure to parenteral antibiotics.11 In our study, the utilization of PAS-COPD might be able to reduce anti-pseudomonal antibiotic overuse from 54.1% to 6.2%. It is suggested that at least 20% of COPD patients hospitalized with CAP might require empirical anti-pseudomonal antibiotic coverage. Also, the prevalence of P.aeruginosa in COPD patients with a score of 0 and 1 points were 2% and 5%, respectively, whereas the prevalence in those with 2 or ≥3 points was 20% and 50%, respectively. For this reason, it would be reasonable not starting antipseudomonal treatment when PAS-COPD≤1 due to the low risk of Pseudomonas etiology. However, a validation score and hospital implementation programs are needed to improve anti-pseudomonal antibiotic overuse in COPD patients with CAP.
The present study has several limitations. Due to multicenter study design, different local standards of care were used. GLIMP is an observational point-prevalence study and was designed to address microbial prevalence and empiric antibiotic use per local standards of care and did not have information about clinical outcomes. For this reason, this study cannot define causal relationships. Otherwise, in over 60% of cases, the etiological agent for the CAP could not be found. Therefore, our results are a representation of what occurs in real life and are similar to prior evidence,40 and this is why our analysis focused on the patients with microbiological testing performed attempting to minimize possible bias. Also, the diagnosis of COPD preceded the hospitalization due to CAP and it was defined according to the GOLD criteria,21 but it was not mandatory to document the FEV1/FVC ratio in the case report form. Similarly, we do not have the thorax CT images to confirm the diagnosis, the severity of bronchiectasis or the doses of inhaled corticosteroids.
In conclusion, microbiological patterns differ according to unique risk factors in COPD patients requiring hospitalization due to CAP. P. aeruginosa represents the most concerning pathogen among COPD patients who developed CAP. Our P. aeruginosa score attempts to assist providers in the appropriate use of empiric anti-pseudomonal therapy and prevent the unnecessary overuse of these antibiotics in COPD patients hospitalized with CAP. Future validation and implementation studies will help us determine the impact of these findings in the routine clinical practice.
Authors’ contributionsSP, FA, JMC, NJS and MIR wrote and edited the manuscript. SA and MIR designed the study and performed statistical analysis. SA, PJM, AR, OS, FS, AU, BM, CNM, MK, and MIR enrolled patients. SA, JG, PJM, AR, OS, FS, GS, AU, BM, CNM, AA and MK contributed intellectually to the final version of the manuscript, and all authors read and approved it.
Written permissionWe give permission for usage of photographs, illustrations, figures, or text from another source.
Financial supportNilam Soni's time is partially funded by the Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1).
Conflict of interestThe content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs. Sergi Pascual Guardia is partially funded by a research mobility grant from Hospital del Mar – IMIM. Judith Marin Corral is partially funded by a research mobility grant from Hospital del Mar – IMIM and Instituto de Salud Carlos III (ISCIII), M-BAE 2019.
We would like to thank the European Respiratory Society, the World Federation of Societies of Intensive and Critical Care Medicine, the American College of Chest Physicians, the Asociación Latinoamericana de Tórax (ALAT), and the Sociedad Argentina de Infectología (SAI) for their support of this project.
We would like to thank the following study contributors for their valuable collaboration:
Argentina: Patricia Karina Aruj, Department of Internal Medicine, University Hospital Alfredo Lanari, Buenos Aires, Argentina; Silvia Attorri, Hospital Luis Lago maggiore, Mendoza, Argentina; Enrique Barimboim, Hospital Central de Mendoza, Argentina; Juan Pablo Caeiro and María I. Garzón, Hospital Privado Universitario, Córdoba, Argentina; Victor Hugo Cambursano, V.H. Dr Cazaux A. Servicio de Neumologia, Hospital Rawson, Córdoba, Argentina; Adrian Ceccato, Hospital Nacional Prof Alejandro Posadas, Argentina; Julio Chertcoff, Florencia Lascar and Fernando Di Tulio, Critical Care Unit and Respiratory Medicine, Buenos Aires British Hospital, Buenos Aires, Argentina; Ariel Cordon Díaz, Hospital General Alvear, Ciudad, Mendoza, Argentina; Lautaro de Vedia, Respiratory Intensive Care Unit, Hospital Muñiz, Buenos Aires, Argentina; Maria Cristina Ganaha, Infectious Diseases Ward, Hospital Interzonal General de Agudos “Vicente Lopez y Planes” from General Rodriguez, Buenos Aires, Argentina; Sandra Lambert, Hospital El Cruce - Alta Complejidad en Red, Argentina; Gustavo Lopardo, Hospital Bernardo Houssay, Vicente López, Argentina; Carlos M. Luna, Pulmonary Medicine Division, Department of Medicine, Hospital de Clínicas, Universidad de Buenos Aires, Argentina; Alessio Gerardo Malberti, Hospital Nuestra Señora del Carmen, Argentina; Nora Morcillo and Silvina Tartara, Hospital Zonal Especializado de Agudos y Crónicos Dr. Antonio A. Cetrangolo, Argentina; Claudia Pensotti, Infectious Diseases and Infection Control Department, Buenos Aires, Clinica Privada Monte Grande, Argentina; Betiana Pereyra, Hospital San Roque, Córdoba, Argentina; Pablo Gustavo Scapellato, Infectious Diseases Department, Hospital D.F. Santojanni, Argentina; Juan Pablo Stagnaro, HZGA Mi Pueblo, Florencio Varela, Argentina. Australia: Sonali Shah, Department of General medicine, Austin hospital, Heidelberg, Australia. Austria: Felix Lötsch and Florian Thalhammer, Division of Infectious Diseases and Tropical Medicine, Department of Medicine I, Medical University of Vienna, Austria. Belgium: Kurt Anseeuw, ZNA Campus Stuivenberg, Antwerp, Belgium; Camille A. Francois, Anesthesia and critical care department, Erasme university hospital, Brussels, Belgium; Eva Van Braeckel, Department of Respiratory Medicine, Ghent University Hospital, Belgium; Jean Louis Vincent, Department of Intensive Care, Erasme University Hospital, Université Libre de Bruxelles, Brussels, Belgium. Benin: Marcel Zannou Djimon, Jules Bashi and Roger Dodo, Centre Hospitalier Universitaire HKM of Cotonou, Benin. Brazil: Simone Aranha Nouér, Federal University of Rio de Janeiro, Brazil. Bulgaria: Peter Chipev and Milena Encheva, Clinic of Pulmonary Diseases, Military Medical Academy, Sofia, Bulgaria; Darina Miteva, UMHAT “St. Marina”, Varna, Bulgaria; Diana Petkova, University Hospital Varna, Bulgaria. Cameroon: Adamou Dodo Balkissou, Yaounde Jamot Hospital, Yaounde, Cameroon; Eric Walter Pefura Yone, Département de Médecine Interne, University of Yaounde, Yaoundé, Cameroon; Bertrand Hugo Mbatchou Ngahane, Douala General Hospital, Douala, Cameroon. China: Ning Shen, Respiratory Medicine, Peking University Third Hospital, Beijing, China; Jin-fu Xu, Department of Respiratory Medicine, Shanghai Pulmonary Hospital, Tongji University, China. Colombia: Carlos Andres Bustamante Rico and Ricardo Buitrago, Clinica Shaio, Bogota, Colombia; Fernando Jose Pereira Paternina, Las Americas Clinic, Medellin, Colombia. Congo: Jean-Marie Kayembe Ntumba, Cliniques Universitaires de Kinshasa, DR Congo. Croatia: Vesna Vladic Carevic, Interne Medicine, Dubrovnik, Croatia; Marko Jakopovic, Medical School, University of Zagreb, Department for Respiratory Diseases Jordanovac, University Hospital Centre Zagreb, Zagreb, Croatia; Mateja Jankovic, University Hospital Center Zagreb, Department for Respiratory Diseases, Zagreb, Croatia; Zinka Matkovic, University Hospital Dubrava, Zagreb, Croatia; Ivan Mitrecic, Karlovac general hospital, Karlovac, Croatia. Denmark: Marie-Laure Bouchy Jacobsson, Emergency Department in North Zealand's Hospital – Hillerød, Denmark; Anette Bro Christensen, Department of Anaethesiology, Viborg Region Hospital, Denmark; Uffe Bødtger, Department of Pulmonology, Naestved Hospital, Denmark; Christian Niels Meyer, Department of Internal Medicine, Roskilde Hospital, Copenhagen University Hospital, Roskilde, Denmark; Andreas Vestergaard Jensen, Gertrud Baunbæk-knudsen, Pelle Trier Petersen and Stine Andersen, Department of Lung- and Infectious Diseases, Nordsjællands Hospital-Hillerød, Denmark. Egypt: Ibrahim El-Said Abd El-Wahhab, Thoracic Medicine, Faculty of Medicine – Mansoura University, Egypt; Nesreen Elsayed Morsy, Pulmonary, Critical Care and Sleep Medicine, Faculty of Medicine, Mansoura University, Mansoura, Egypt; Hanaa Shafiek, Chest diseases department, Faculty of Medicine, Alexandria University, Egypt; Eman Sobh, Chest Diseases Department, Al-Azhar University, Cairo, Egypt. Ethiopia: Kedir Abdella Abdulsemed, Department of Medical Laboratory Science and Pathology, College of Health sciences, Mycobacteriology Research Centre, Institute of Biotechnology Research, Jimma University, Jimma, Ethiopia. France: Fabrice Bertrand, Critical care Unit, Robert Ballanger Hospital, Aulnay sous Bois, France; Christian Brun-Buisson, Univ Hospital Henri Mondor, 94000 Créteil, France; Etienne de Montmollin, Intensive care unit, Hôpital Delafontaine, Centre hospitalier de Saint-Denis, Saint-Denis, France; Muriel Fartoukh, Unité de réanimation médico-chirurgicale, Pôle Thorax Voies aériennes, Hôpital Tenon, Groupe Hospitalier Est Parisien, France; Jonathan Messika, Publique-Hôpital de Paris, Service de Réanimation Médico-chirurgicale, Hôpital Louis Mourier, Colombes, France, and Université Paris Diderot, IAME, UMR 1137, Sorbonne Paris Cité, Paris, France; Pierre Tattevin, Infectious Diseases &ICU, Pontchaillou University Hospital, Rennes, France; Abdo Khoury, Department of Emergency Medicine & Critical Care, University of Franche – Comté, Medical Center, France. Gambia: Bernard Ebruke, Medical Research Council Unit, Gambia. Germany: Michael Dreher, Department of Cardiology, Pneumology, Vascular Medicine and Intensive Care Medicine, University Hospital Aachen, Aachen, Germany; Martin Kolditz, Division of Pulmonology, Medical Department I, University Hospital Carl Gustav Carus, Technische Universität Dresden, Germany; Matthias Meisinger, Klinikum Niederlausitz GmbH, Klinik für Innere Medizin und Intensivmedizin, Senftenberg, Germany; Mathias W. Pletz and Stefan Hagel, Center for Infectious Diseases and Infection Control, Jena University Hospital, Germany; Jan Rupp, Department of Molecular and Infectious Diseases, University of Lübeck, Lübeck, Germany; Tom Schaberg, Zentrum für Pneumologie, Agaplesion Diakonieklinikum Rotenburg, Germany; Marc Spielmanns, Internal Medicine Department, Pulmonary rehabilitation and Department of Health, School of Medicine, University Witten-Herdecke, St.Remigius-Hospital, Leverkusen, Germany; Petra Creutz and Norton Suttorp, Department of Infectious Disease and Respiratory Medicine, Charité – University Medicine, Berlin, Germany. Ghana: Beatrice Siaw-Lartey, Komfo-Anokye Teaching Hospital, Kumasi, Ghanuffa. Greece: Katerina Dimakou, 5th Respiratory Medicine Dpt, “SOTIRIA” Chest Hospital, Athens 11527, Greece; Mina Gaga, 7th Resp. Med. Dept and Asthma Center, Athens Chest Hospital, Grece; Dimosthenis Papapetrou, Medical Group of Athens (Paleo Faliro Clinic), Athens, Greece; Evdoxia Tsigou and Dimitrios Ampazis, Agioi Anargiroi Hospital, Kifissia, Athens, Greece; Evangelos Kaimakamis, Intensive Care Unit, “G. Papanikolaou” General Hospital of Thessaloniki, Greece. India: Mohit Bhatia, S.S. Hospital IMS BHU Varanasi, India; Raja Dhar, Fortis Hospitals, Kolkata, India; George D'Souza, Department of Pulmonary Medicine, St. John's Medical College Hospital, Bangalore, India, 560034; Rajiv Garg, Department of Respiratory Medicine, King George's Medical University UP, Lucknow, India; Parvaiz A Koul, Department of Internal & Pulmonary Medicine, SheriKashmir Institute of Medical Sciences, Srinagar, India; PA Mahesh and BS Jayaraj, Department of Pulmonary Medicine, JSS Medical College, JSS University, Mysore, India; Kiran Vishnu Narayan, Pulmonary Medicine, Government Medical College Kozhikode, Kerala, India; Hirennappa B Udnur and Shashi Bhaskara Krishnamurthy, Columbia Asia Hospital, Hebbal, Bengaluru, Karnataka, India; Surya Kant, Department of Respiratory Medicine, King George's Medical University, Chowk, Lucknow 226003, Uttar Pradesh, India; Rajesh Swarnakar, Getwell Hospital & Research Institute, Dhantoli, Nagpur, India; Sneha Limaye and Sundeep Salvi, on behalf of the Respiratory Research Network of India (RRNI) from the Chest Research Foundation in Pune, India. Iran: Keihan Golshani, Isfahan University of Medical Sciences; Iran. Ireland: Vera M Keatings, Letterkenny General Hospital, Co. Donegal, Ireland; Ignacio Martin-Loeches, Multidisciplinary Intensive Care Research Organization (MICRO), St James's University Hospital, Trinity Centre for Health Sciences Dublin, Ireland. Israel: Yasmin Maor, Infectious Disease Unit, Affiliated to Tel Aviv University, Wolfson Medical Center, Holon, Israel; Jacob Strahilevitz, Department of Clinical Microbiology & Infectious Diseases, Hadassah-Hebrew University, Jerusalem, Israel. Italy: Salvatore Battaglia, University of Palermo, Pneumologia DiBiMIS, Palermo, Italy; Maria Carrabba, Internal Medicine Department, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122, Milano, Italy; Piero Ceriana, Pulmonary rehabilitation, IRCCS Fondazione Maugeri, 27100, Pavia, Italy; Marco Confalonieri, Department of Pulmonology, University Hospital, Trieste, Italy; Antonella d’Arminio Monforte, Department of Health Sciences, Clinic of Infectious Disease, San Paolo Hospital, University of Milan, Italy; Bruno Del Prato, Interventional Pneumology, Hospital Antonio Cardarelli, Naples, Italy; Marino De Rosa, UOC Pneumologia P.O. San Filippo Neri ASL RM E Roma, Italy; Riccardo Fantini, Respiratory Diseases clinic, Policlinico di Modena, 41124 Modena, Italy; Paola Faverio, Cardio-Thoracic-Vascular Department, University of Milan Bicocca, Respiratory Unit, San Gerardo Hospital, ASST di Monza, Monza, Italy; Giuseppe Fiorentino, UOC Fisiopatologia e Riabilitazione Respiratoria AO Ospedali dei Colli PO Monaldi, Italy; Maria Antonia Gammino, Pulmonary Medicine Unit, San Martino Hospital, ASL 5 Oristano, Sardegna, Italy; Francesco Menzella, Department of Cardiac-Thoracic-Vascular and Intensive Care Medicine, Pneumology Unit, IRCCS-Arcispedale Santa Maria Nuova, Reggio Emilia, Italy; Giuseppe Milani, Azienda Ospedaliera Sant Anna di Como, Presidio Ospedale S. Anna Nuovo, Unità Operativa di Pneumologia, Como, Italy; Stefano Nava, Alma Mater University of Bologna, DIMES, Respiratory and Critical Care Unit Sant’Orsola Malpighi Hospital, Italy; Gerardo Palmiero, Respiratory Unit, Versilia Hospital, Azienda USL 12 Viareggio, Lido di Camaiore, Lucca, Italy; Roberta Petrino and Barbara Gabrielli, Emergency Medicine Unit, S. Andrea Hospital, Vercelli, Italy; Paolo Rossi, Internal Medicine Department, Azienda Ospedaliero-Universitaria S. Maria della Misericordia, Udine, Italy; Claudio Sorino, Pulmonology Unit, A.O. Sant’Anna di Como, Italy; Gundi Steinhilber, Spedali Civili Brescia, U.O. Pneumologia e Fisiopatologia Respiratoria, Brescia, Italy; Alessandro Zanforlin, ULSS 18 Rovigo, Ospedale San Luca, 45027 Trecenta (RO), Italy; Fabio Franzetti, Manuela Carugati, Manuela Morosi and Elisa Monge, Department of Biomedical and Clinical Sciences, Division of Infectious Diseases, Luigi Sacco Hospital, Università degli Studi di Milano, Milan, Italy; Mauro Carone, Fondazione Salvatore Maugeri, IRCCS, Cassano Murge, Italy; Vincenzo Patella, Allergology and Clinical Immunology Unit, Department of Medical Sciences, Battipaglia Hospital, Battipaglia, Salerno, Italy; Simone Scarlata, Geriatrics, Unit of Respiratory Pathophysiology and Thoracic Endoscopy, Campus Bio Medico University and Teaching Hospital, Rome, Italy; Andrea Comel, UO Pneumologia, Ospedale Pederzoli, Peschiera del Garda, Italy. Japan: Kiyoyasu Kurahashi, Yokohama City University Medical Center, Japan. Lebanon: Zeina Aoun Bacha, Medicine school, St Joseph University, Beyrouth, Lebanon. Mexico: Daniel Barajas Ugalde, National Institute of Respiratory Diseases, Mexico; Omar Ceballos Zuñiga, Hospital General de Mexicali, Mexicali, Baja California, Mexico; José F Villegas, Hospital Universitario Monterrey, n. l. México CP 64030. Montenegro: Milic Medenica, Hospital for Lung Diseases – Brezovik, Niksic, Montenegro. Netherlands: E.M.W. van de Garde, Dept. Clinical Pharmacy, St. Antonius Hospital, Utrecht/Nieuwegein, The Netherlands. Nepal: Deebya Raj Mihsra, Internal Medicine, BP Koirala Institute of Health Sciences, Nepal; Poojan Shrestha, Oxford University Clinical Research Unit, Patan Hospital, Nepal; New Zealand: Elliott Ridgeon, Medical Research Institute of New Zealand; Nigeria: Babatunde Ishola Awokola, Department of Family Medicine & Primary Care, Lily Hospitals Limited, Warri, Nigeria; Ogonna N.O. Nwankwo, University of Calabar Teaching Hospital, Calabar, Nigeria; Adefuye Bolanle Olufunlola, Olabisi Onabanjo University teaching hospital, Sagamu, Ogun State, Nigeria; Segaolu Olumide, Department of Medicine (Pulmonary Unit), University College Hospital, Ibadan, Nigeria; Kingsley N. Ukwaja, Department of Medicine, Federal Teaching Hospital Abakaliki, Ebonyi State, Nigeria; Pakistan: Muhammad Irfan, Section of Pulmonary and Critical Care Medicine, Department of Medicine, Aga Khan University, Karachi-74800, Pakistan; Poland: Lukasz Minarowski, Department of Lung Diseases and Tuberculosis, Medical University of Bialystok, Poland; Skoczyński Szymon, Department of Pneumology, School of Medicine in Katowice, Medical University of Silesia, Katowice, Institute of Occupational Medicine and Environmental Health, Sosnowiec, Poland; Portugal: Felipe Froes, Hospital Pulido Valente – CHLN, Lisboa, Portugal; Pedro Leuschner, Centro Hospitalar do Porto, Porto, Portugal; Mariana Meireles, Cláudia Ferrão, Pedro Leuschner and João Neves, Serviço de Medicina, Centro Hospitalar do Porto, Largo Prof. Abel Salazar, 4099-001 Porto, Portugal; Sofia B Ravara, Faculty of Health Sciences, University of Beira Interior; Cova da Beira Hospital Center, 6200-251 Covilhã, Portugal; Republic of Moldova: Victoria Brocovschii, Department of Pneumology & Allergology, State University of Medicine and Pharmacy “Nicolae Testemitanu” Republic of Moldova; Chesov Ion, Clinic of Anesthesia and Intensive Care “Valeriu Ghrerg”, Institute of Emergency Medicine, State University of Medicine and Pharmacy “Nicolae Testemitanu”, Chisinau, Republic of Moldova; Doina Rusu, SMFU “N.Testemitanu”, Chisinau, Republic of Moldova; Cristina Toma, Department of Pneumology & Allergology, State University of Medicine and Pharmacy “Nicolae Testemitanu”, Chisinau, Republic of Moldova; Romania: Daniela Chirita, Hospital Sfantul Stefan, Bucharest, Romania; Carmen Mihaela Dorobat, Universitatea de Medicină şi Farmacie “Gr. T. Popa” I a ş i Facultatea de Medicină Stomatologică, Spitalul Clinic de Boli Infecţioase “Sfânta Parascheva” I a ş i str. Octav Botez, nr. 2, 700116, Iaşi, Romania; Russia: Alexei Birkun, Department of Anesthesiology, Critical Care and Emergency Medicine, Medical Academy named after S. I. Georgievsky, Russian Federation; Anna Kaluzhenina, Volgograd State Medical University, Russia. Saudi Arabia: Abdullah Almotairi, King Fahad medical City (KFMC), Riyadh, KSA; Zakeya Abdulbaqi Ali Bukhary, College of Medicine, Taibah University, Medina, KSA; Jameela Edathodu, Al Faisal University, King Faisal Specialist Hospital, Riyadh, KSA; Amal Fathy, Pulmonary and respiratory critical care Medicine, Mansoura University Egypt, Affiliate at Taibah University, KSA; Abdullah Mushira Abdulaziz Enani and Nazik Eltayeb Mohamed, Infectious Diseases Section, Medical Specialties Department, King Fahad Medical City, Riyadh, KSA; Jawed Ulhadi Memon, Pulmonology Division, Department of Internal Medicine, King Fahad Hospital, Hofuf, Al Ahasa, 31982, KSA; Abdelhaleem Bella, Dammam University-Saudi Arabia and King Fahad Hospital, KSA. Serbia: Nada Bogdanović, Pulmonary department of KHC Dr. Dragiša Mišović, Belgrade, Serbia; Branislava Milenkovic, Clinic for Pulmonary Diseases, Clinical Centre of Serbia, Faculty of Medicine, University of Belgrade, Belgrade, Serbia; Dragica Pesut, University of Belgrade School of Medicine, Teaching Hospital of Pulmonology, Clinical Centre of Serbia, Belgrade, Serbia. Spain: Luis Borderìas, Respiratory and Sleep Unit, Hospital San Jorge, Huesca, Spain; Noel Manuel Bordon Garcia, Barcelona Policlínic and Moises Broggi Hospital at sant Joan Despí, Spain; Hugo Cabello Alarcón, Sant Hospital Seu de Urgell, Catalonia, Spain; Catia Cilloniz and Antoni Torres, Department of Pneumology, Institut Clinic del Tórax, Hospital Clinic of Barcelona, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), University of Barcelona (UB), Spain; Vicens Diaz-Brito and Xavier Casas, Infectious diseases Unit and Pneumology Service, Parc Sanitari Sant Joan de Deu, Sant Boi, Barcelona, Spain; Alicia Encabo González, Hospital Complex of Pontevedra, Spain; Maria Luisa Fernández-Almira, Medicina Interna, Hospital Universitario Central de Asturias, Spain; Miguel Gallego, Department of Respiratory Medicine, Hospital de Sabadell, Institut Universitari Parc Taulí-UAB, Sabadell, Spain. CIBER de Enfermedades Respiratorias, CIBERES, Bunyola, Spain; Inmaculada Gaspar-GarcÍa, Department of Respiratory Medicine, Hospital Costa del Sol, Marbella, Málaga, Spain; Juan González del Castillo, Emergency Department, Hospital Universitario Clínico San Carlos, Madrid, Spain; Patricia Javaloyes Victoria, Hospital General Universitario de Alicante, Alicante, Spain; Elena Laserna Martínez, Hospital Mollet, Barcelona, Spain; Rosa Malo de Molina, University Hospital Puerta de Hierro Majadahonda, Madrid; Pedro J Marcos, Servicio de Neumología, Complejo Hospitalario Universitario de A Coruña (CHUAC), INIBIC, Sergas, Universidade de A Coruña (UDC), Spain; Rosario Menéndez, Pneumology Service, Universitary and Polytechnic Hospital La Fe, Valencia, Spain; Ana Pando-Sandoval, Hospital Universitario Central de Asturias. Area de Gestion Clinica de Pulmon. Servicio de Neumologia, Oviedo, Spain; Cristina Prat Aymerich, Alicia Lacoma de la Torre and Ignasi García-Olivé, Microbiology Department and Pneumology Department, Hospital Universitari Germans Trias i Pujol, Institut d’Investigació Germans Trias i Pujol, Badalona, Spain. Universitat Autònoma de Barcelona. CIBER Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, Spain; Jordi Rello and Silvia Moyano, Critical Care Department, Hospital Vall d’Hebron, Barcelona, Spain; Francisco Sanz, Servicio de Neumología, Consorci Hospital General Universitari de Valencia, Valencia, Spain; Oriol Sibila and Ana Rodrigo-Troyano, Servei de Pneumologia, Hospital de la Santa Creu i Sant Pau, IIB-Sant Pau, Barcelona, Spain; Jordi Solé-Violán, Hospital Universitario de Gran Canaria Dr Negrín, Las Palmas de Gran Canaria, Spain; Ane Uranga, Pulmology Department, Hospital of Galdakao-Usansolo, Spain; Job FM van Boven, Hospital Universitari Son Espases, Palma de Mallorca, Spain; Ester Vendrell Torra and Jordi Almirall Pujol, Intensive Care Medicine, Hospital de Mataró, Spain. South Africa: Charles Feldman, Division of Pulmonology, Department of Internal Medicine, Charlotte Maxeke Johannesburg Academic Hospital, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa. South Korea: Ho Kee Yum, Inje Univ. Seoul Paik Hospital, South Korea. Togo: Arnauld Attannon Fiogbe, Pulmonology and Infectious Diseases Service/University hospital of Sylvanus Olympio, Lomé, Togo. Tunisia: Ferdaous Yangui, Department of Pneumology, Hospital of Internal Forces Security (I.F.S), Marsa, Tunis, Tunisia. Turkey: Semra Bilaceroglu, Izmir Dr. Suat Seren Training and Research Hospital for Thoracic Medicine and Surgery, Izmir, Turkey; Levent Dalar, Pulmonary Medicine, Istanbul Bilim University, Istanbul, Turkey; Ufuk Yilmaz, Suat Seren Chest Disease and Surgery Training and Research Hospital, İzmir, Turkey. Ukraine: Artemii Bogomolov, Vinnitsa National Pirogov Memorial Medical University, Vinnitsa regional antituberculosis hospital, Vinnitsa, Ukraine. United Arab Emirates: Naheed Elahi, Dubai Hospital, U.A.E. United Kingdom: Devesh J Dhasmana, Victoria Hospital, Kirkcaldy, NHS Fife, UK; Andrew Feneley, Rhiannon Ions, Julie Skeemer and Gerrit Woltmann, University Hospitals of Leicester NHS Trust and University of Leicester, Leicester, UK; Carole Hancock, Royal Respiratory Research Team, Royal Liverpool University Hospital, Liverpool, UK; Adam T Hill, Royal Infirmary and University of Edinburgh, UK; Banu Rudran, The Royal London Hospital, Barts Health Trust, London, UK; Silvia Ruiz-Buitrago and Marion Campbell, Hairmyres Hospital, Eaglesham Road, East Kilbride, G75 8RG, UK; Paul Whitaker, Department of Respiratory Medicine, St James's Hospital, Leeds, LS9 7TF, UK; Alexander Youzguin, Southport and Ormskirk Hospitals NHS Trust, UK; Anika Singanayagam, Imperial College Healthcare NHS Trust, London, UK. United States of America: Karen S Allen, University of Oklahoma Health Sciences Center, USA; Veronica Brito, Texas A&M Health Science Center, Division of Pulmonary, Critical Care and Sleep Medicine Baylor Scott & White Health, USA; Jessica Dietz, Fargo VA Health Care System, Fargo, North Dakota, USA; Claire E. Dysart and Susan M. Kellie, Clement J. Zablocki VA Medical Center, 5000 W. National Ave Milwaukee, WI 53295, USA, Division of Infectious Diseases, University of New Mexico School of Medicine, Raymond G. Murphy VA Medical Center, 1501 San Pedro SE Albuquerque, NM87108, USA; Ricardo A Franco-Sadud and Garnet Meier, Division of Hospital Medicine, Cook County Hospital, Chicago, USA; Thomas L. Holland and Stephen P. Bergin, Department of Medicine, Duke University Medical Center and School of Medicine, Duke Clinical Research Institute, USA; Fayez Kheir, Department of Pulmonary Diseases, Critical Care & Environmental Medicine, Tulane University Health Sciences Center, New Orleans, LA, USA; Mark Landmeier, Division of Pulmonary and Critical Care Medicine, Northwestern Memorial Hospital, Chicago, IL 60611, USA; Manuel Lois, John Peter Smith Hospital, Fort Worth, TX, 76104, USA; Girish B Nair, Interstitial Lung Disease Program and Pulmonary Rehabilitation, SUNY Stony Brook Winthrop University Hospital, Mineola, NY 115501, USA; Hemali Patel, Department of Medicine, Division of General Internal Medicine, Hospital Medicine Group, University of Colorado, USA; Katherine Reyes, Henry Ford Hospital, Detroit, IL, USA; William Rodriguez-Cintron, Pulmonary/Critical Care Medicine VA Caribbean Healthcare System, USA; Shigeki Saito, Tulane University, New Orleans, USA; Nilam J. Soni, Julio Noda, Cecilia I. Hinojosa, Stephanie M. Levine, Luis F. Angel, Luis F. Reyes, and Antonio Anzueto, Divisions of Hospital Medicine & Pulmonary/Critical Care Medicine, South Texas Veterans Health Care System, University of Texas Health Science Center San Antonio, San Antonio, TX, USA; K. Scott Whitlow, John Hipskind, Kunal Sukhija and Vicken Totten, Kaweah Delta Health Care District, Department of Emergency Medicine, Visalia, CA, USA; Richard G. Wunderink and Ray D. Shah, Northwestern University Feinberg School of Medicine, Chicago, IL, USA. Zambia: Kondwelani John Mateyo, Department of Internal Medicine, University Teaching Hospital, Lusaka, Zambia. Other investigators: Lorena Noriega; Ezequiel Alvarado; Mohamed Aman; Lucía Labra.