While the beneficial effects of physical fitness on general health are well-documented, the specific relationship between different types of physical fitness, particularly cardiorespiratory fitness (CRF) and muscular endurance fitness (MEF), and lung function in physically active young adults remains less explored.
ObjectiveThis study investigated the relationship between CRF and MEF, and their correlation with lung function in physically active young adults.
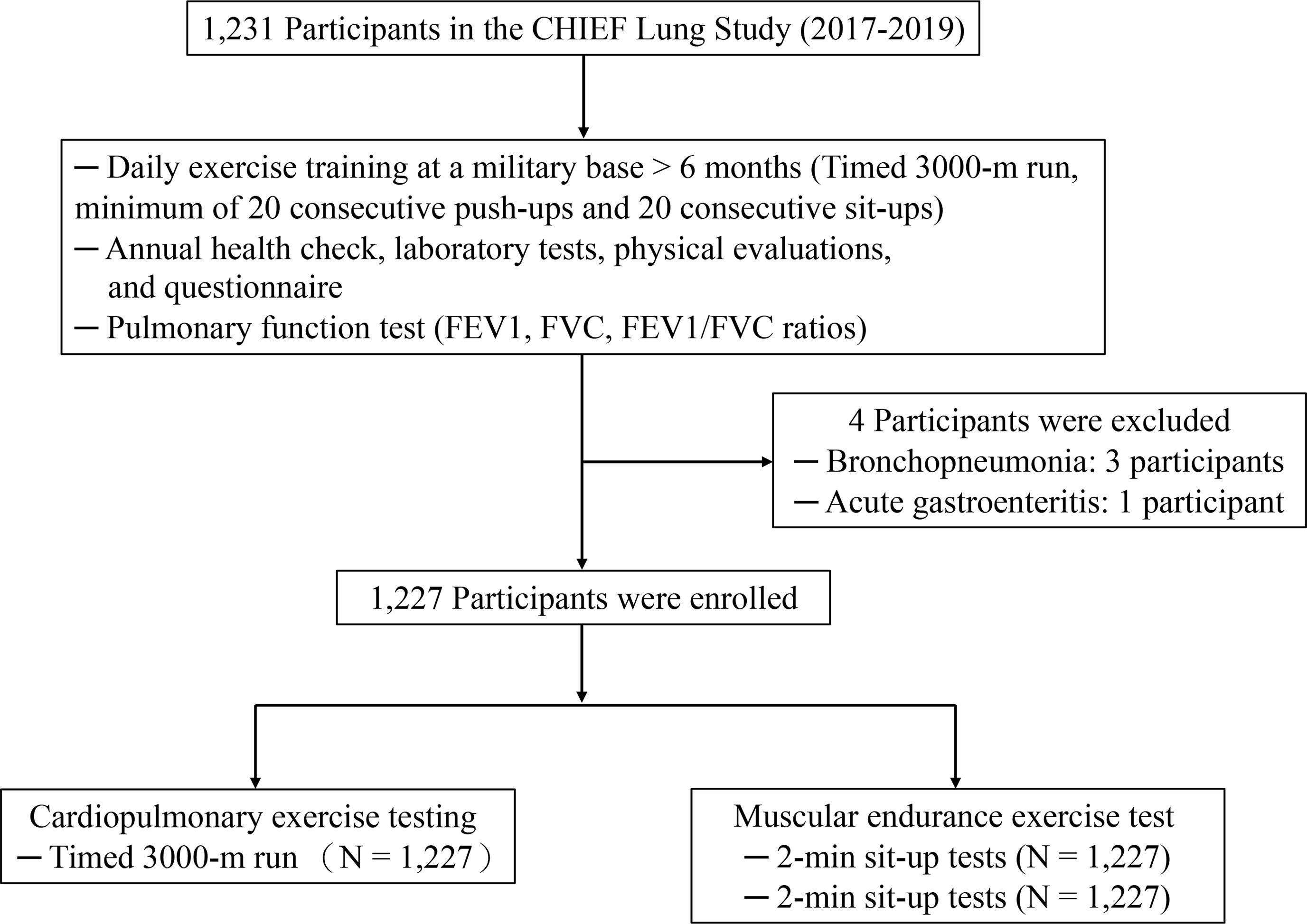
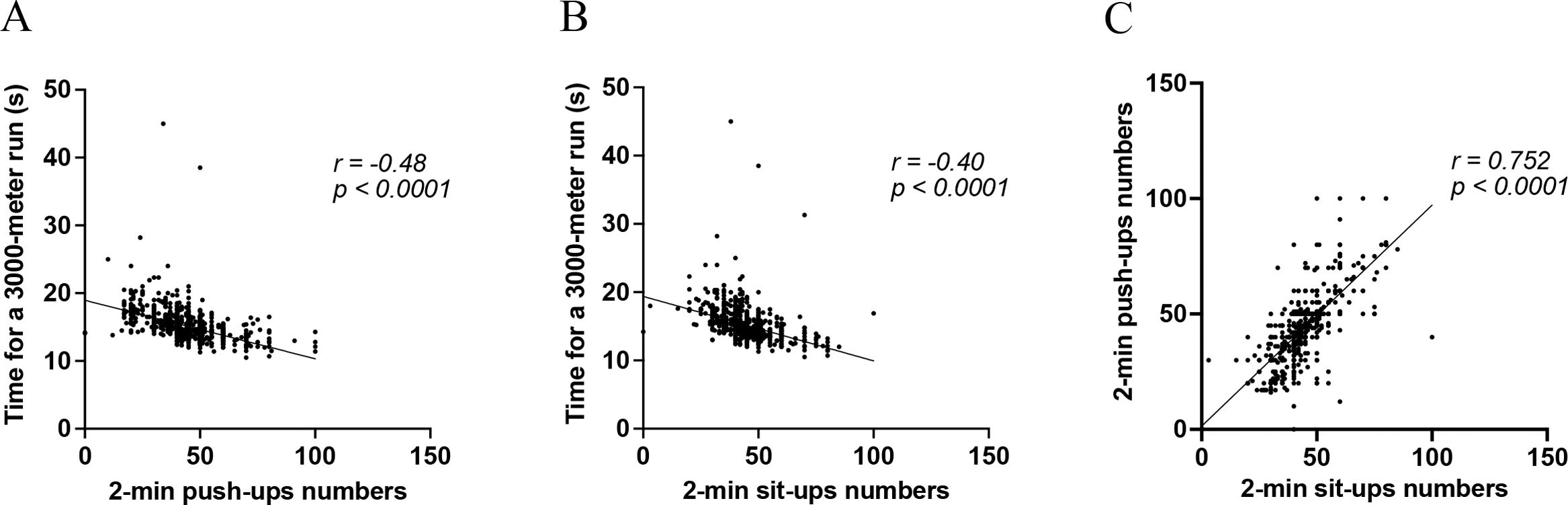
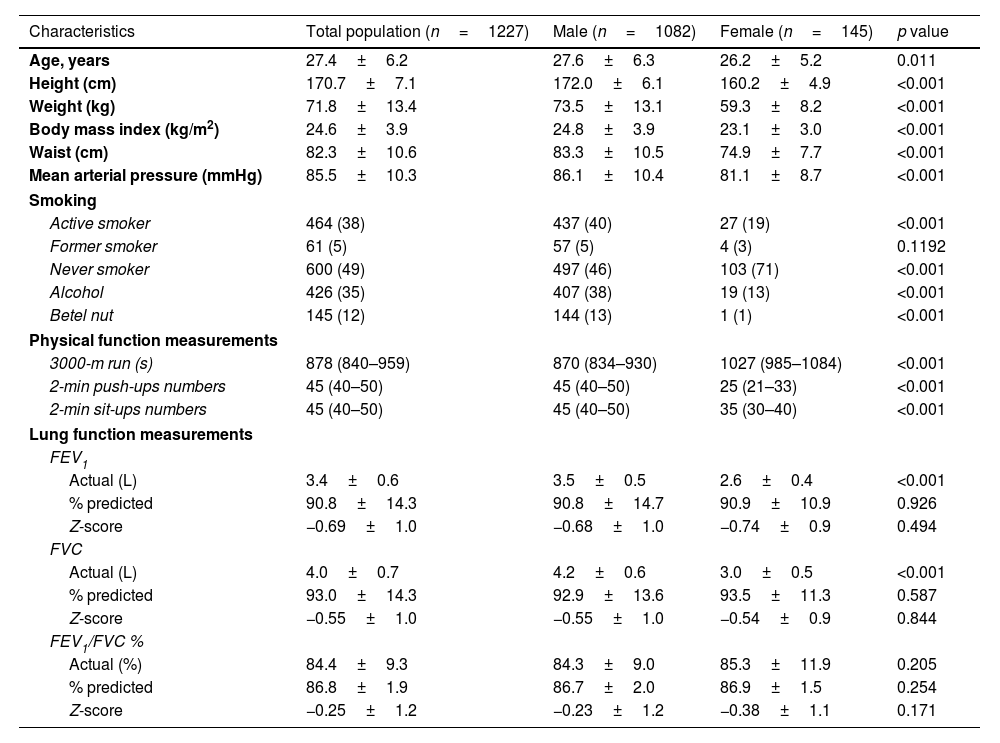
MethodsThis cross-sectional study involved a cohort of 1227 physically active young adults without lung diseases. Lung function was assessed using FEV1, FVC, and FEV1/FVC measurements. The 3000-m run was used to assess CRF, and the 2-min push-up and sit-up tests were used to assess MEF. Multivariable linear regression analysis was used to evaluate the relationships between these fitness measures and lung function, adjusting for potential covariates.
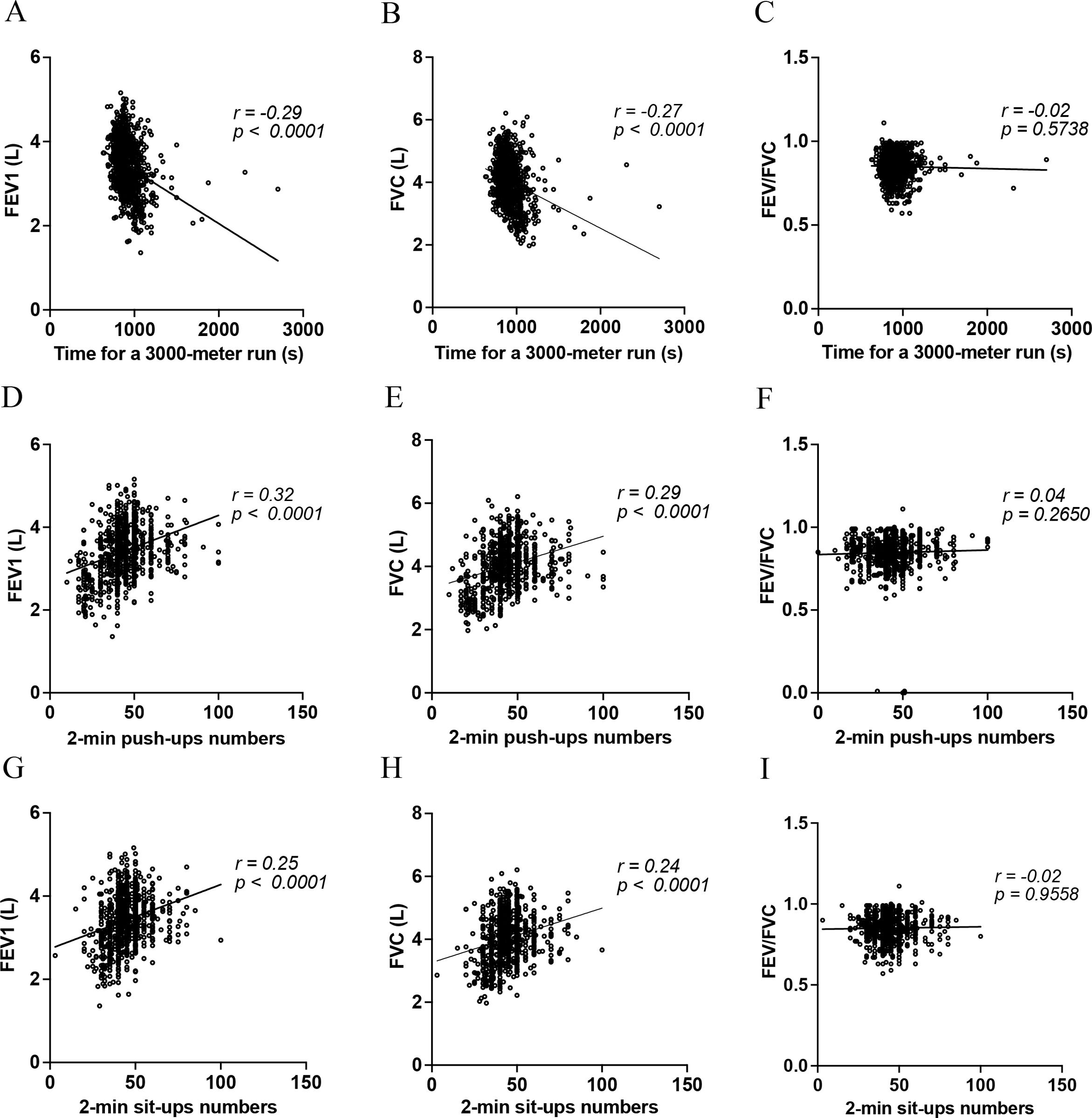
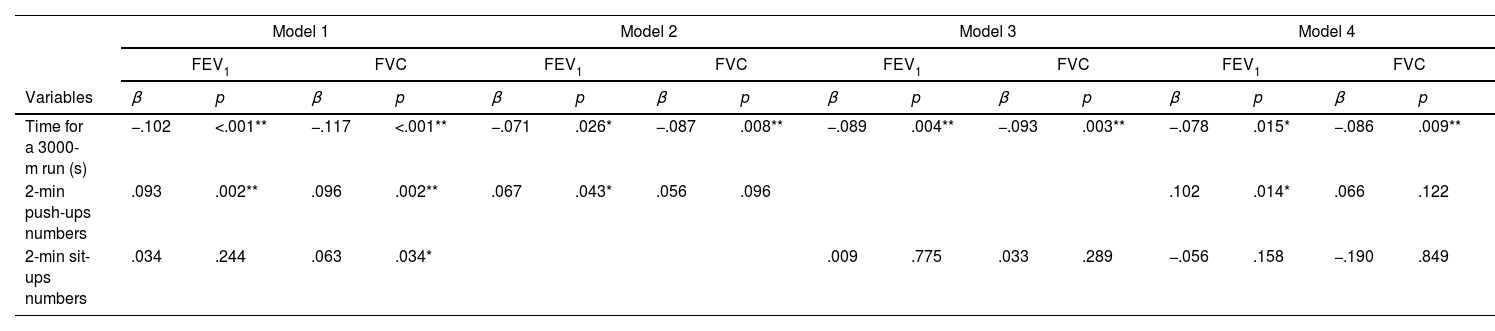
ResultsEnhanced CRF was associated with superior FEV1 and FVC after adjusting for covariates (β=−.078, p=.015 for FEV1; β=−.086, p=.009 for FVC). Push-ups were positively associated with FEV1 (β=.102, p=.014), but not with FVC. In contrast, sit-ups showed no significant correlation with lung function in the fully adjusted model.
ConclusionThe study demonstrated a clear association between improved physical fitness and better lung function in physically active young adults, with various exercises showing distinct associations with lung metrics. Notably, push-ups were particularly associated with higher FEV1. A future prospective study is necessary to determine whether routine exercises, such as push-ups, might lead to greater lung function.