The aim of this study was to analyze the impact of occupational exposure on chronic obstructive pulmonary disease (COPD) and respiratory symptoms in the general Spanish population.
MethodsThis was a study nested in the Spanish EPISCAN II cross-sectional epidemiological study that included participants who had completed a structured questionnaire on their occupational history, a questionnaire on respiratory symptoms, and forced spirometry. The data were analyzed using Chi-square and Student's t tests and adjusted models of multiple linear regression and logistic regression.
ResultsWe studied 7502 subjects, 51.1% women, with a mean age of 60±11 years. Overall, 53.2% reported some respiratory symptoms, 7.9% had respiratory symptoms during their work activity, 54.2% were or had been smokers, and 11.3% (851 subjects) met COPD criteria on spirometry. A total of 3056 subjects (40.7%) reported exposure to vapors, gases, dust or fumes (VGDF); occupational exposure to VGDF was independently associated with the presence of COPD (OR 1.22, 95% CI: 1.03–1.44), respiratory symptoms (OR 1.45, 95%: CI 1.30–1.61), and respiratory symptoms at work (OR 4.69, 95% CI: 3.82–5.77), with a population attributable fraction for COPD of 8.2%.
ConclusionsOccupational exposure is associated with a higher risk of COPD and respiratory symptoms in the Spanish population. These results highlight the need to follow strict prevention measures to protect the respiratory health of workers.
Chronic obstructive pulmonary disease (COPD) is the leading cause of respiratory death and the third overall cause of death worldwide after ischemic heart disease and cerebrovascular disease.1 Genetic and environmental factors are involved in its pathogenesis, and of these, smoking is the greatest risk factor. However, in both the United States2 and Europe,3,4 about 25% of the adult population with COPD are never-smokers, so the cause of COPD in these individuals needs to be investigated.
Occupational exposure has a major impact on the respiratory health of the population, given the large number of potentially affected people. Most studies have been conducted in populations in the US,5–7 China,8 and in some countries in central9,10 and northern Europe.11–14 Aside from some discrepancies, an association between occupational exposure, COPD risk, and the development of respiratory symptoms has been demonstrated in these populations. Differences in effects may be due to individual factors, differences in work activity and working conditions in different countries. The population attributable fraction (PAF) of occupational exposure in COPD prevalence has been estimated at around 20% in smokers and 31% in non-smokers.15 Occupational exposure has also been linked to the presence of respiratory symptoms, such as cough and chronic bronchitis.16–20
The risk of occupational exposure to respiratory health in Mediterranean populations is little known, and only two studies have been published in the Spanish population. One used a job-exposure matrix to show that exposure to high levels of biological dust was associated with symptoms of chronic bronchitis and worse spirometry results in young people between 20 and 44 years of age from five autonomous communities.21 In a second study, occupational exposure to dust, fumes and gases was associated with symptoms of chronic bronchitis and airflow obstruction in an industrialized region of Catalonia.22
The aim of this study was to analyze the impact of occupational exposure on the risk of developing respiratory symptoms and COPD in a representative sample of the general Spanish population that included subjects of all ages from all regions of Spain enrolled in the third study of COPD prevalence in Spain (EPISCAN II).4
Materials and MethodsDesignThis was a sub-study of the multicenter cross-sectional EPISCAN II study that included 9433 randomly selected subjects of both sexes aged over 40 years from 17 Spanish autonomous communities who agreed to attend visits in 20 hospitals. The aim of the EPISCAN II was to determine the prevalence of COPD in Spain; the methodology has been described elsewhere.23
Study VariablesSociodemographic variables, smoking, use of e-cigarettes, and other patterns of tobacco use and previous exposure to biomass fumes were recorded. The European Coal and Steel Community (ECCS) questionnaire on respiratory symptoms was administered.24 Work-related respiratory symptoms were assessed using two questions from this questionnaire25,26: Have you ever noticed shortness of breath, wheezing or chest tightness at work? Have you ever had to change or quit your job because it affected your breathing?
Post-bronchodilator spirometry was performed according to the criteria of the American Thoracic Society/European Respiratory Society (ATS/ERS).27 COPD was defined as the presence of a post-bronchodilator FEV1/FVC ratio of less than 0.728
An occupational exposure questionnaire adapted from the Spanish version of the European Community Respiratory Health Survey (ECRHS)19 was administered. This included: (1) a list of all jobs performed in chronological order; (2) exposure to any of the 18 substances in an attached list; and (3) engagement in any of 22 separately listed occupations. These lists contained substances and occupations previously associated with the development of COPD and the presence of work-related symptoms (Annex 1). Based on previous studies,6,14,29 occupational exposure was defined as an affirmative answer to the question Have you ever been exposed to vapors, gases, dust or fumes at work? or to the question on self-reported exposure to any substance in the attached list. Biomass exposure was defined as an affirmative answer to the question Have you had or have regular contact with smoke from wood or logs (e.g. fireplaces/wood stoves, work activity related to wood burning, etc.)?
Statistical AnalysisThe mean and standard deviation and the number and percentage of participants by response category were used for the description of continuous and categorical variables, respectively. Comparisons between the characteristics of patients who completed the occupational questionnaire and those who did not were made using the Chi-square test for categorical variables and Student's t for quantitative variables. Individuals exposed or not exposed to VGDF were compared using the Chi-square test. The threshold for statistical significance was 0.05.
Several multivariate logistic regression models were performed to estimate the association between occupational exposure and the presence of COPD or respiratory symptoms. In an initial analysis, the diagnosis of COPD was evaluated as a dependent variable, and exposure to VGDF, the substances to which the participants could have been exposed, and their occupations were evaluated as independent variables. A second analysis examined respiratory symptoms (according to the ECCS) as a dependent variable, and VGDF exposure, substances and occupations as independent variables. Adjustment variables incorporated in each model were age, sex, educational level, body mass index, smoking and accumulated exposure in pack-years, and exposure to biomass smoke. COPD was added to the risk model for respiratory symptoms as an adjustment variable. The detected associations are expressed as odds ratios (OR) with their 95% confidence intervals. The PAF is an estimate of the proportion of all cases of a disease in a given population that would not have occurred in the absence of the exposure of interest. To estimate the proportion of the prevalence of COPD attributable to occupational exposures, the PAF was estimated from a multiple logistic regression analysis following the Greenland and Drescher method,30 adjusted for sex, age, body mass index, smoking, level of education, and exposure to biomass smoke, as used in studies with similar methodologies to ours. The formula adopted was: PAF=(exposure prevalence×OR/1+exposure prevalence×OR)×100.5,14
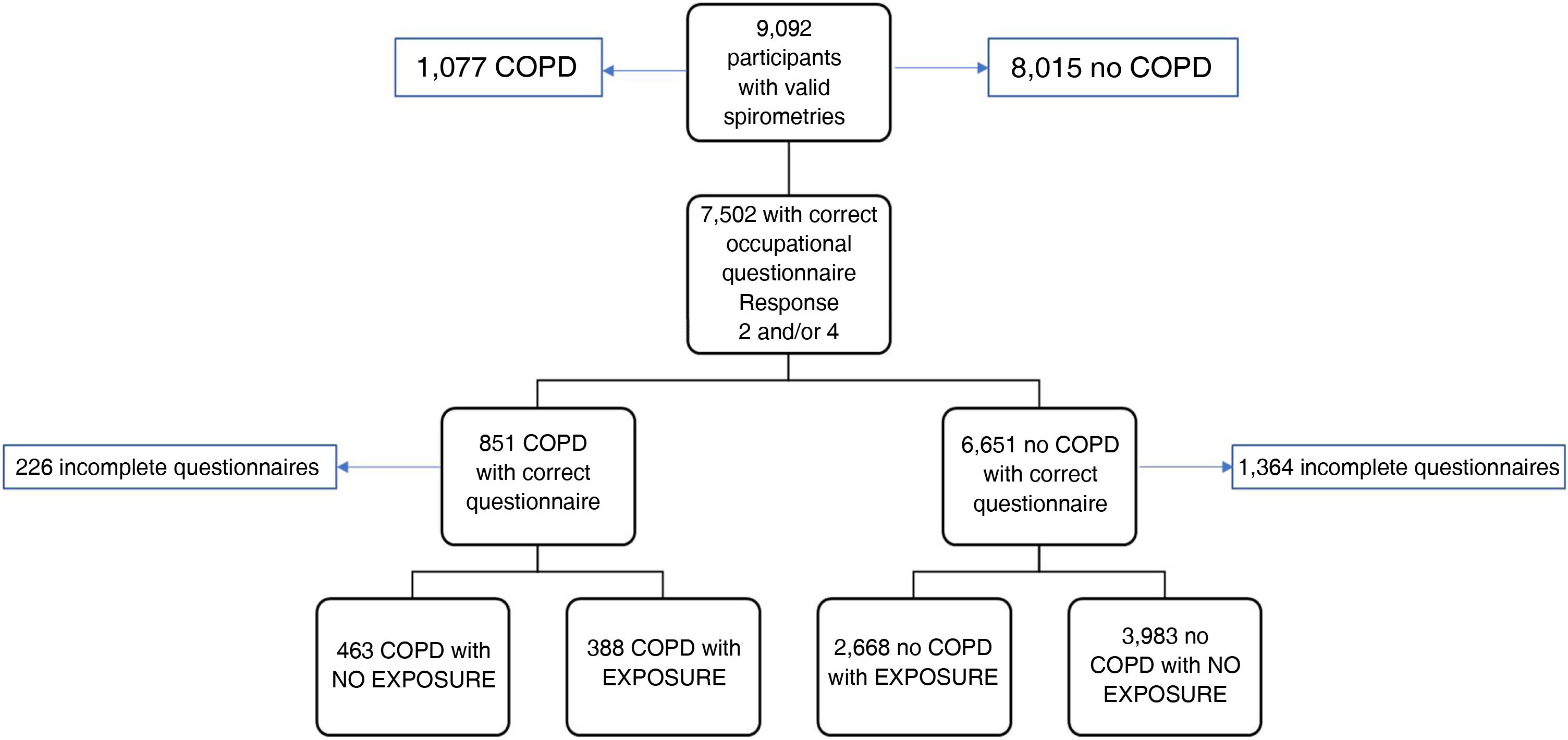
ResultsOf the 9092 patients with valid spirometry included in the EPISCAN II study,4 1590 cases were excluded because the occupational exposure questionnaire was not properly completed. Therefore, a total of 7502 individuals of both sexes were included (Fig. 1), with a mean age of 60±11 years, of which 3831 (51.1%) were women and 55.6% were educated to higher than primary school level. One third (34.7%) were former smokers, 19.9% were active smokers, and 17.2% had been exposed at some point in their life to biomass smoke. The characteristics of excluded subjects are shown in Table S1 of the Supplement.
Flow diagram of study participants distributed according to COPD diagnosis and occupational exposure.
Exposure was defined as an affirmative answer to the question Have you ever been exposed to vapors, gases, dust or fumes at work? or at least one positive response to the list of substances in the questionnaire.
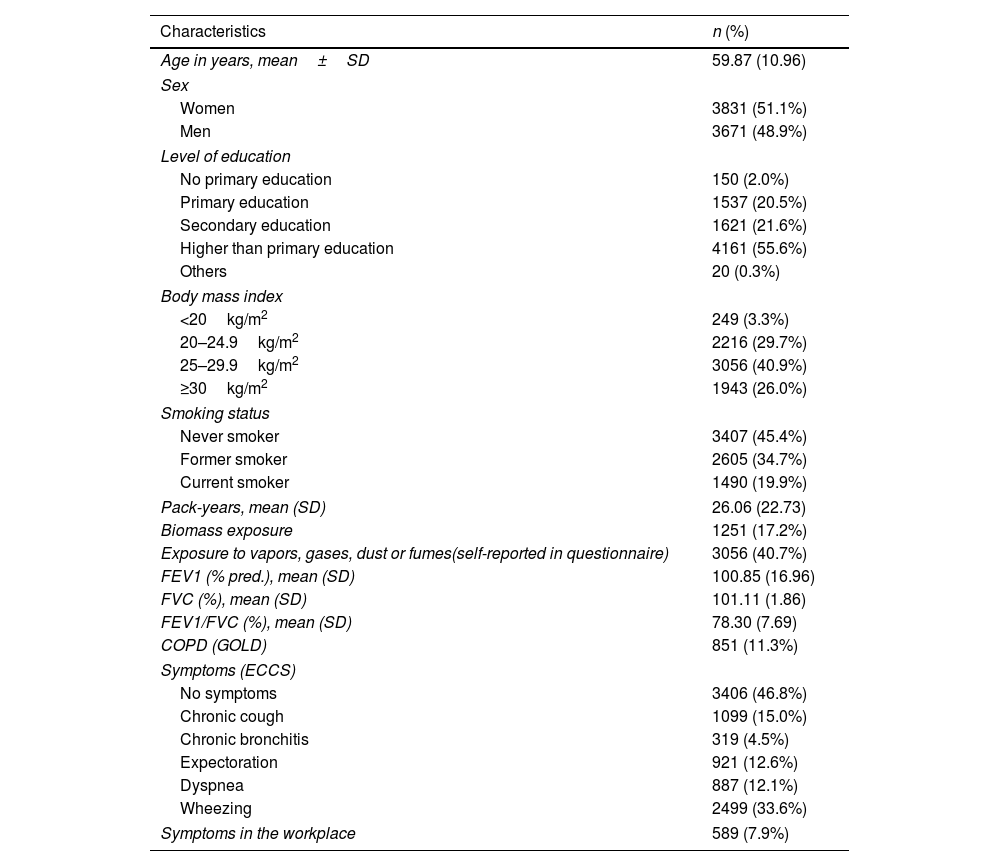
Overall, 3056 (40.7%) subjects reported exposure to VGDF, 851 (11.3%) met COPD criteria on spirometry, and 53.2% reported experiencing respiratory symptoms, the most frequent being wheezing (33.6%), cough (15%), and expectoration (12.6%). A total of 7.9% of the subjects experienced respiratory symptoms during their work activity (Table 1).
Demographic, Clinical and Spirometry Characteristics of the Study Population (n=7502).
| Characteristics | n (%) |
|---|---|
| Age in years, mean±SD | 59.87 (10.96) |
| Sex | |
| Women | 3831 (51.1%) |
| Men | 3671 (48.9%) |
| Level of education | |
| No primary education | 150 (2.0%) |
| Primary education | 1537 (20.5%) |
| Secondary education | 1621 (21.6%) |
| Higher than primary education | 4161 (55.6%) |
| Others | 20 (0.3%) |
| Body mass index | |
| <20kg/m2 | 249 (3.3%) |
| 20–24.9kg/m2 | 2216 (29.7%) |
| 25–29.9kg/m2 | 3056 (40.9%) |
| ≥30kg/m2 | 1943 (26.0%) |
| Smoking status | |
| Never smoker | 3407 (45.4%) |
| Former smoker | 2605 (34.7%) |
| Current smoker | 1490 (19.9%) |
| Pack-years, mean (SD) | 26.06 (22.73) |
| Biomass exposure | 1251 (17.2%) |
| Exposure to vapors, gases, dust or fumes(self-reported in questionnaire) | 3056 (40.7%) |
| FEV1 (% pred.), mean (SD) | 100.85 (16.96) |
| FVC (%), mean (SD) | 101.11 (1.86) |
| FEV1/FVC (%), mean (SD) | 78.30 (7.69) |
| COPD (GOLD) | 851 (11.3%) |
| Symptoms (ECCS) | |
| No symptoms | 3406 (46.8%) |
| Chronic cough | 1099 (15.0%) |
| Chronic bronchitis | 319 (4.5%) |
| Expectoration | 921 (12.6%) |
| Dyspnea | 887 (12.1%) |
| Wheezing | 2499 (33.6%) |
| Symptoms in the workplace | 589 (7.9%) |
The excluded subjects were younger, fewer were men, and they had a lower body mass index and a lower prevalence of COPD and respiratory symptoms compared to participants (Supplement Table S1). Individuals with occupational exposure to VGDF were younger, fewer were educated to university level, and a higher proportion were smokers. Overall, 58.4% of those exposed were or had been active smokers compared to 51% who were never-smokers (p<0.001) (Supplement Table S2).
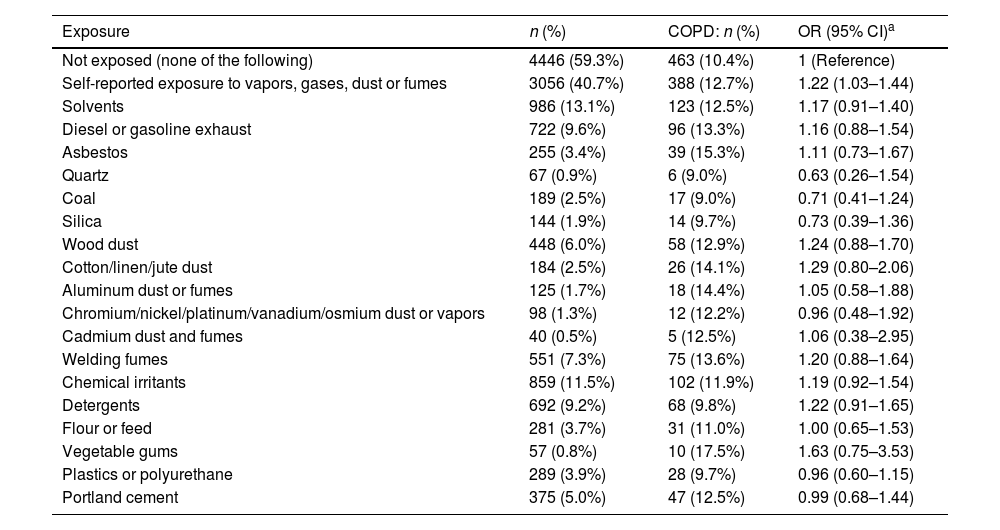
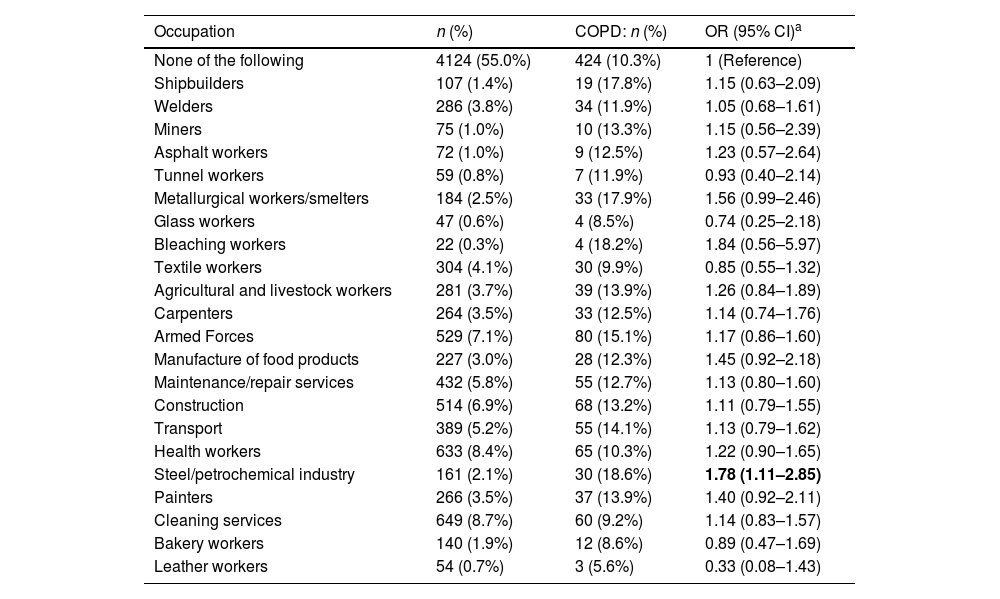
The most common substances to which the study population had been exposed were solvents (13.1%), chemical irritants (11.5%), diesel or gasoline (9.6%), and detergents (9.2%); when considered individually, no significant differences in exposure were observed between subjects with and without COPD, bearing in mind that the number of subjects exposed to any particular substance was relatively low. The most common occupations were cleaning staff (8.7%), health workers (8.4%), and the armed forces (7.1%) (Table 2).
Association Between Lifetime Occupational Exposure (Substances) and COPD. Multivariate Logistic Regression (n=7502).
| Exposure | n (%) | COPD: n (%) | OR (95% CI)a |
|---|---|---|---|
| Not exposed (none of the following) | 4446 (59.3%) | 463 (10.4%) | 1 (Reference) |
| Self-reported exposure to vapors, gases, dust or fumes | 3056 (40.7%) | 388 (12.7%) | 1.22 (1.03–1.44) |
| Solvents | 986 (13.1%) | 123 (12.5%) | 1.17 (0.91–1.40) |
| Diesel or gasoline exhaust | 722 (9.6%) | 96 (13.3%) | 1.16 (0.88–1.54) |
| Asbestos | 255 (3.4%) | 39 (15.3%) | 1.11 (0.73–1.67) |
| Quartz | 67 (0.9%) | 6 (9.0%) | 0.63 (0.26–1.54) |
| Coal | 189 (2.5%) | 17 (9.0%) | 0.71 (0.41–1.24) |
| Silica | 144 (1.9%) | 14 (9.7%) | 0.73 (0.39–1.36) |
| Wood dust | 448 (6.0%) | 58 (12.9%) | 1.24 (0.88–1.70) |
| Cotton/linen/jute dust | 184 (2.5%) | 26 (14.1%) | 1.29 (0.80–2.06) |
| Aluminum dust or fumes | 125 (1.7%) | 18 (14.4%) | 1.05 (0.58–1.88) |
| Chromium/nickel/platinum/vanadium/osmium dust or vapors | 98 (1.3%) | 12 (12.2%) | 0.96 (0.48–1.92) |
| Cadmium dust and fumes | 40 (0.5%) | 5 (12.5%) | 1.06 (0.38–2.95) |
| Welding fumes | 551 (7.3%) | 75 (13.6%) | 1.20 (0.88–1.64) |
| Chemical irritants | 859 (11.5%) | 102 (11.9%) | 1.19 (0.92–1.54) |
| Detergents | 692 (9.2%) | 68 (9.8%) | 1.22 (0.91–1.65) |
| Flour or feed | 281 (3.7%) | 31 (11.0%) | 1.00 (0.65–1.53) |
| Vegetable gums | 57 (0.8%) | 10 (17.5%) | 1.63 (0.75–3.53) |
| Plastics or polyurethane | 289 (3.9%) | 28 (9.7%) | 0.96 (0.60–1.15) |
| Portland cement | 375 (5.0%) | 47 (12.5%) | 0.99 (0.68–1.44) |
Of the 851 participants with COPD who completed a valid questionnaire, 388 (45.6%) had a history of VGDF exposure: the difference with respect to COPD subjects with no exposure was statistically significant (p<0.0022). Occupational exposure to VGDF was independently associated with COPD (OR 1.22, 95% CI: 1.03–1.44) (Table 2). Among the specific occupations, only the steel and petrochemical industries were associated with COPD (OR 1.78, 95% CI: 1.11–2.85) (Table 3). The association between occupational exposure to VGDF and COPD, with an OR of 1.22 and a prevalence of occupational exposure of 40.7%, gives an estimated PAF% of 8.2% (95% CI: 1.2–15).
Association Between Occupational Exposure (Jobs) and COPD. Multivariate Logistic Regression (n=7502).
| Occupation | n (%) | COPD: n (%) | OR (95% CI)a |
|---|---|---|---|
| None of the following | 4124 (55.0%) | 424 (10.3%) | 1 (Reference) |
| Shipbuilders | 107 (1.4%) | 19 (17.8%) | 1.15 (0.63–2.09) |
| Welders | 286 (3.8%) | 34 (11.9%) | 1.05 (0.68–1.61) |
| Miners | 75 (1.0%) | 10 (13.3%) | 1.15 (0.56–2.39) |
| Asphalt workers | 72 (1.0%) | 9 (12.5%) | 1.23 (0.57–2.64) |
| Tunnel workers | 59 (0.8%) | 7 (11.9%) | 0.93 (0.40–2.14) |
| Metallurgical workers/smelters | 184 (2.5%) | 33 (17.9%) | 1.56 (0.99–2.46) |
| Glass workers | 47 (0.6%) | 4 (8.5%) | 0.74 (0.25–2.18) |
| Bleaching workers | 22 (0.3%) | 4 (18.2%) | 1.84 (0.56–5.97) |
| Textile workers | 304 (4.1%) | 30 (9.9%) | 0.85 (0.55–1.32) |
| Agricultural and livestock workers | 281 (3.7%) | 39 (13.9%) | 1.26 (0.84–1.89) |
| Carpenters | 264 (3.5%) | 33 (12.5%) | 1.14 (0.74–1.76) |
| Armed Forces | 529 (7.1%) | 80 (15.1%) | 1.17 (0.86–1.60) |
| Manufacture of food products | 227 (3.0%) | 28 (12.3%) | 1.45 (0.92–2.18) |
| Maintenance/repair services | 432 (5.8%) | 55 (12.7%) | 1.13 (0.80–1.60) |
| Construction | 514 (6.9%) | 68 (13.2%) | 1.11 (0.79–1.55) |
| Transport | 389 (5.2%) | 55 (14.1%) | 1.13 (0.79–1.62) |
| Health workers | 633 (8.4%) | 65 (10.3%) | 1.22 (0.90–1.65) |
| Steel/petrochemical industry | 161 (2.1%) | 30 (18.6%) | 1.78 (1.11–2.85) |
| Painters | 266 (3.5%) | 37 (13.9%) | 1.40 (0.92–2.11) |
| Cleaning services | 649 (8.7%) | 60 (9.2%) | 1.14 (0.83–1.57) |
| Bakery workers | 140 (1.9%) | 12 (8.6%) | 0.89 (0.47–1.69) |
| Leather workers | 54 (0.7%) | 3 (5.6%) | 0.33 (0.08–1.43) |
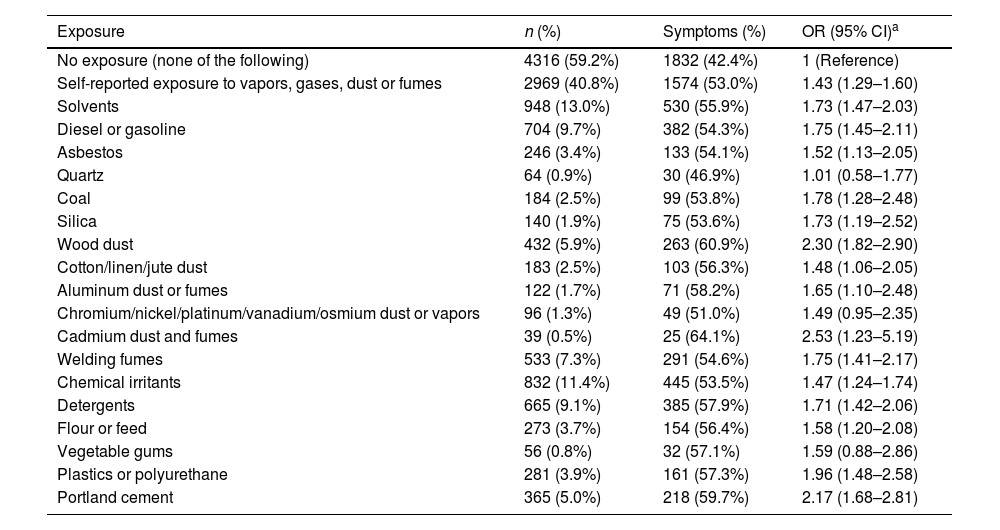
The relationship between occupational exposure and respiratory symptoms was analyzed in the 7285 participants who completed the ECCS questionnaire correctly: of these, 2969 (40.8%) had VGDF exposure. In the multivariate analysis, occupational exposure to VGDF was associated with the presence of some respiratory symptoms (OR 1.43, 95% CI: 1.29–1.60) (Table 4). Significant associations were detected between most of the substances studied and the presence of respiratory symptoms, the strongest being with cadmium dust and fumes (OR 2.53, 95% CI: 1.23–5.19), wood dust (OR 2.30, 95% CI: 1.82–2.90), and cement (OR 2.17, 95% CI: 1.68–2.81). Exposure to VGDF was associated with specific symptoms such as cough (OR 1.50, 95% CI: 1.30–1.74), chronic bronchitis (OR 1.59, 95% CI: 1.23–2.04), expectoration (OR 1.50, 95% CI: 1.28–1.76), dyspnea (OR 1.33, 95% CI: 1.13–1.56), and wheezing (OR 1.48, 95% CI: 1.32–1.65). VGDF exposure was associated with most occupations, while the OR was higher or statistically significant in the case of employees in the bleaching industry (OR 2.96, 95% CI: 1.06–8.30), painters (OR 2.06, 95% CI: 1.53–2.77), and asphalt workers (OR 2.01, 95% CI: 1.15–3.52) (Supplement Table S4). All substances and professions studied (except bleach works) were associated with symptoms in the workplace. The substances that carried the highest risk were vegetable gums (OR 12.19, 95% CI: 6.31–23.55), aluminum (OR 10.19, 95% CI: 6.31–16.44), silica (OR 9.99, 95% CI: 6.25–15.96), and coal (OR 9.54, 95% CI: 6.19–14.71), while the occupations with the highest risk were mining (OR 6.47, 95% CI: 3.39–12.36), shipbuilding (OR 6.11, 95% CI: 3.44–10.86), and the petrochemical and steel industries (OR 6.06, 95% CI: 3.86–9.50) (Supplement Tables S3a and S3b.
Association Between Lifetime Occupational Exposure (Substances) and COPD and Respiratory Symptoms. Multivariate Logistic Regression (n=7285).
| Exposure | n (%) | Symptoms (%) | OR (95% CI)a |
|---|---|---|---|
| No exposure (none of the following) | 4316 (59.2%) | 1832 (42.4%) | 1 (Reference) |
| Self-reported exposure to vapors, gases, dust or fumes | 2969 (40.8%) | 1574 (53.0%) | 1.43 (1.29–1.60) |
| Solvents | 948 (13.0%) | 530 (55.9%) | 1.73 (1.47–2.03) |
| Diesel or gasoline | 704 (9.7%) | 382 (54.3%) | 1.75 (1.45–2.11) |
| Asbestos | 246 (3.4%) | 133 (54.1%) | 1.52 (1.13–2.05) |
| Quartz | 64 (0.9%) | 30 (46.9%) | 1.01 (0.58–1.77) |
| Coal | 184 (2.5%) | 99 (53.8%) | 1.78 (1.28–2.48) |
| Silica | 140 (1.9%) | 75 (53.6%) | 1.73 (1.19–2.52) |
| Wood dust | 432 (5.9%) | 263 (60.9%) | 2.30 (1.82–2.90) |
| Cotton/linen/jute dust | 183 (2.5%) | 103 (56.3%) | 1.48 (1.06–2.05) |
| Aluminum dust or fumes | 122 (1.7%) | 71 (58.2%) | 1.65 (1.10–2.48) |
| Chromium/nickel/platinum/vanadium/osmium dust or vapors | 96 (1.3%) | 49 (51.0%) | 1.49 (0.95–2.35) |
| Cadmium dust and fumes | 39 (0.5%) | 25 (64.1%) | 2.53 (1.23–5.19) |
| Welding fumes | 533 (7.3%) | 291 (54.6%) | 1.75 (1.41–2.17) |
| Chemical irritants | 832 (11.4%) | 445 (53.5%) | 1.47 (1.24–1.74) |
| Detergents | 665 (9.1%) | 385 (57.9%) | 1.71 (1.42–2.06) |
| Flour or feed | 273 (3.7%) | 154 (56.4%) | 1.58 (1.20–2.08) |
| Vegetable gums | 56 (0.8%) | 32 (57.1%) | 1.59 (0.88–2.86) |
| Plastics or polyurethane | 281 (3.9%) | 161 (57.3%) | 1.96 (1.48–2.58) |
| Portland cement | 365 (5.0%) | 218 (59.7%) | 2.17 (1.68–2.81) |
This study shows that occupational exposure is independently associated with a higher risk of COPD and respiratory symptoms among the Spanish population. Specific associations were also identified between COPD and the steel and petrochemical industries and between respiratory symptoms and a range of substances and occupations. An association between occupational exposure and symptoms manifested during work also emerged.
The impact of occupational exposure in southern Europe has not been fully characterized. This study is the largest conducted to date in the Mediterranean area. Two previous Spanish studies have been reported: one was limited to an industrialized region of Catalonia22 and the other included subjects under 44 years of age from 5 autonomous communities.21 Our study is the first nationwide study with a large sample of subjects of both sexes and a wide age range that is representative of the population as a whole.
The association of occupational exposure to VGDF with COPD, with an OR of 1.22, confirms the risk of certain occupations to the respiratory health of our population. This finding is comparable to that reported in a recent meta-analysis,31 which estimated a global OR of 1.43, a rate slightly lower than that of studies conducted in the United States and northern Europe that reported ORs ranging from 1.6129 to 2.0.5 In our study, the PAF% – or estimated reduction in the proportion of COPD in exposed subjects if the exposure is eliminated – was 8.2%. This estimate varies widely in different studies and its interpretation may be limited in transversal designs such as ours. Some studies based on self-administered questionnaires to determine VGDF exposure have reported higher PAF% values: for example, 17.4%, 20% and 37%,5,14,32 while others reported similar results to ours, such as 9% in Catalonia22 and 10% in China,33 and as low as 5.2% in the Philippines34 and 3.9% in another region of China.35 An ATS/ERS consensus document estimated that the PAF% for exposure to VGDF would be around 14.1%.36 These differences could be due to several factors, such as methodological differences or the characteristics of the population samples, for example, a case–control design or the inclusion of subjects in certain age ranges.14 Another important factor is the percentage of exposed individuals in each population sample studied. Exposure in our study was 40.7%, a percentage comparable to that of similar studies, such as 33%–58% in a European study,18 35% in a US series,29 and 52% in Catalonia.22 These values show that levels of occupational exposure are similar in industrialized countries. Our study offers interesting insights into a little-known factor, namely, the specific association of the steel/petrochemical industry with COPD, as previously observed in a French study.37
Our results indicate that occupational exposure to VGDF is directly associated with general respiratory symptoms (OR 1.45) and with specific symptoms such as cough, chronic bronchitis, expectoration, dyspnea and wheezing (OR 1.3–1.5). These results are consistent with a study in men exposed to VGDF29 that found an OR for cough, expectoration and wheezing of 1.83, 1.84 and 2.01, respectively, and a study conducted in a section of the Spanish population that showed an OR of 2.1 and 1.5 for cough and expectoration, respectively. Another finding worth noting is the association between respiratory symptoms and most of the substances and occupations included in the questionnaire. The agents associated with the highest risks were wood dust and cement, substances to which the working population is commonly exposed.
The association of occupational exposure to VGDF and specific substances and jobs with the appearance of respiratory symptoms in the workplace is a very interesting result of this study and one that has not been reported to date. High risks with ORs of between 6 and 7 have been detected for the steel, petrochemical, shipbuilding, and mining industries. This association underlines the relationship between occupational exposure and respiratory disease. The results mentioned above also suggest that the appearance of these symptoms can indirectly influence work absenteeism and reduce productivity. In fact, there is evidence that exposure to VGDF is associated with absenteeism due to respiratory disease,38 and that people with COPD and asthma have a higher frequency of absenteeism, which affects labor productivity.39
This study has several limitations. Firstly, it is a cross-sectional study, so we cannot determine the course of exposure or establish a causal relationship with COPD and respiratory symptoms. However, we do not believe that a hypothetical change in employment would alter our findings, as patients were questioned about exposures at any time in their lifetime, and a question was included about the need to change occupations due to respiratory symptoms. Secondly, the exclusion of 1590 subjects who had not properly completed their occupational exposure questionnaire may have led to an inclusion bias, and we cannot determine if this possible bias led to an over- or under-estimation of the associations detected. These subjects were older and had a higher prevalence of COPD, so their exclusion is unlikely to weaken the associations. Thirdly, the existence of a memory bias that would lead patients with COPD and/or respiratory symptoms to overestimate previous occupational exposures or symptoms cannot be ruled out. This bias is inevitable in any study based on self-reported exposure. On the other hand, our study has obvious strengths, primarily, in our opinion, its population design with the inclusion of a large number of randomly selected subjects that clearly represent the Spanish population. The rigor of the EPISCAN II study, within which this study is nested, in which post-bronchodilator spirometry was performed according to the highest quality control standards in hospitals throughout the 17 Spanish autonomous communities, ensures the validity of the information on the respiratory health of the participants. The use of a structured occupational exposure questionnaire is another strong point of the study. The questionnaire, in addition to the list of occupations, included a list of professions and risk substances that according to the previous literature, had been associated with COPD; this questionnaire, therefore, offers a detailed overview of the participants’ self-reported occupational exposure. Finally, the high number of adjustment variables, especially the adjustment for tobacco exposure, strengthens the multivariate analyses and increases the strength of the associations found.
ConclusionsThis study shows that occupational exposure is associated with COPD and respiratory symptoms in the Spanish population. The results support the available evidence on the need to follow strict prevention measures in our work environment to avoid affecting respiratory health. In the future, this impact should be evaluated progressively in longitudinal studies.
Ethical ApprovalAll participants gave written informed consent to participate in the study.
The study was approved by the ethics committee (EC) of each of the participating centers, with the EC of Hospital Universitario La Princesa acting as the reference committee. The EPISCAN II protocol is registered in https://clinicaltrials.gov under no. NCT03028207 and in www.gsk-clinicalstudyregister.com/study/205932.
FundingBoth this study and EPISCAN II were sponsored by GSK.
Conflict of InterestEduardo Loeb has received honoraria for speaking engagements, attendance at congresses and consultancy from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim and Pfizer. Jean-Paul Zock has no conflict of interest. Marc Miravitlles has received honoraria for speaking engagements and consultancy from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Laboratorios Esteve, Gebro Pharma, Kamada, GlaxoSmithKline, Grifols, Menarini, Mereo Biopharma, Novartis, pH Pharma, Palobiofarma SL, Rovi, Teva, Spin Therapeutics, Verona Pharma and Zambon, and research grants from Grifols. Juan José Soler-Catalonia has received honoraria for speaking engagements from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GlaxoSmithKline, Menarini, Novartis, Teva, and consultancy fees from AstraZeneca, Bial, Boehringer Ingelheim, GlaxoSmithKline, Ferrer and Novartis. Joan B. Soriano has no conflict of interest. Francisco García-Rio has received honoraria for speaking engagements and consultancy from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Novartis, Pfizer and Rovi, and research grants from Chiesi, Esteve, Gebro Pharma, GlaxoSmithKline, Menarini and Teva. Pilar de Lucas has no conflict of interest. Inmaculada Alfageme has no conflict of interest. Ciro Casanova has received honoraria for speaking engagements and consultancy from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Novartis, and research grants from GlaxoSmithKline, Menarini and AstraZeneca. José Miguel Rodríguez González-Moro has no conflict of interest. Julio Ancochea has received honoraria for speaking engagements and consultancy from Actelion, Air Liquide, Almirall, AstraZeneca, Boehringer Ingelheim, Carburos Médica, Chiesi, Faes Farma, Ferrer, GlaxoSmithKline, InterMune, Linde Healthcare, Menarini, MSD, Mundipharma, Novartis, Pfizer, Roche, Rovi, Sandoz, Takeda and Teva. Borja G. Cosio has received honoraria for speaking engagements and consultancy from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Novartis, Teva, and research grants from GlaxoSmithKline, Menarini and AstraZeneca. Jaume Ferrer has received honoraria for speaking engagements, attendance at congresses and consultancy from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Chiesi and Novartis. Esther Rodríguez has no conflict of interest.
The EPISCAN II study was funded by an unrestricted grant from GlaxoSmithKline. The authors are very grateful to Monica Sarmiento and Neus Canal from IQVIA, and GlaxoSmithKline for their collaboration in this study.
The following researchers participated in the EPISCAN II study:
Scientific Committee: Inmaculada Alfageme, Pilar de Lucas, Julio Ancochea, Marc Miravitlles, Juan José Soler-Cataluña, Francisco García-Río, Ciro Casanova, José Miguel Rodríguez González-Moro, Borja G. Cosio, Guadalupe Sánchez, and Joan B Soriano
Principal investigators, sub-investigators and participating centers: