Alpha1-antitrypsin deficiency (AATD) is a rare medical condition and a potential treatable cause of bronchiectasis.1 AATD is caused by biallelic variants of SERPINA1 gene and is associated with reduced secretion, circulating levels or activity of alpha1-antitrypsin (AAT). More than 100 SERPINA1 disease-related variants have been reported, but the most prevalent one in Europeans is rs28929474, encoding for the PiZ AAT variant.2 The antiprotease AAT inhibits serine proteases in the lungs and its deficiency worsens neutrophilic inflammation, which is a typical pathogenetic feature of bronchiectasis.2 The pharmacological administration of AAT may prevent bronchiectasis progression thought modulation of inflammation.3 The first prevalence study on genetic variants of SERPINA1 in patients with bronchiectasis was performed 24 years ago in France and did not detect any significant differences in the distribution of AAT alleles between cases and controls.4 Another UK study showed that AATD rate was low in bronchiectasis.5,6 However, the epidemiology of SERPINA1 variants in patients with bronchiectasis living in Southern Europe is currently unknown. The aim of this study was, therefore, to evaluate the frequency of PiZ and PiS variants (i.e., most frequent causes of AATD) in a cohort of Italian bronchiectasis patients.
An observational, cross-sectional study was conducted between March 2017 and July 2019. Consecutive patients with clinically – (daily sputum production) and radiologically proved bronchiectasis were enrolled. Patients with either cystic fibrosis (CF) or traction bronchiectasis due to pulmonary fibrosis or those undergoing long-term inhaled antibiotic therapy were excluded. Patients with chronic obstructive pulmonary disease (COPD) were excluded according to Araújo objective algorithm for bronchiectasis aetiology diagnosis.7 Only subjects in stable clinical conditions (≥1 month apart from the last exacerbation and antibiotic course) were included. Concomitantly, consecutive healthy blood donors were enrolled between May and November 2020. All subjects were aged ≥18 years and European. The study was approved by the local ethical committee (#660, October 14th, 2014, Ethical Committee Milano Area B), and all subjects provided a written informed consent. Both patients and controls underwent genetic screening for SERPINA1 gene. SERPINA1 variants in bronchiectasis patients were evaluated using a custom panel of all coding exons and exon-intron boundaries of 82 selected genes (Parseq Lab.) through an Illumina MiSeq platform. Then, the analysis was focused specifically on the SERPINA1 gene (NM_000295.4). Sequencing was performed with MiSeq Reagent Kit v3 and t MiSeq Reagent Kit v2. Raw data were analyzed by the VariFind software. Genetic variants were filtered using MAF<1% in 1000 Genomes Project as a cut off. Global Screening Array (GSA), version 2.0 (Illumina), was used to genotype the control group. The PiZ variant (rs28929474) 14:g.94378610C>T was directly genotyped while PiS variant (rs17580) 14.g.94380925T>A has been imputed. The role of rs28929474 and rs17580 variants allele dosage (additive genetic model) on the risk of bronchiectasis was tested by a logistic regression model. Statistical analysis and phenotypic association studies were performed using R software (R version 4.0.3). p-Values<0.05 were considered statistically significant.
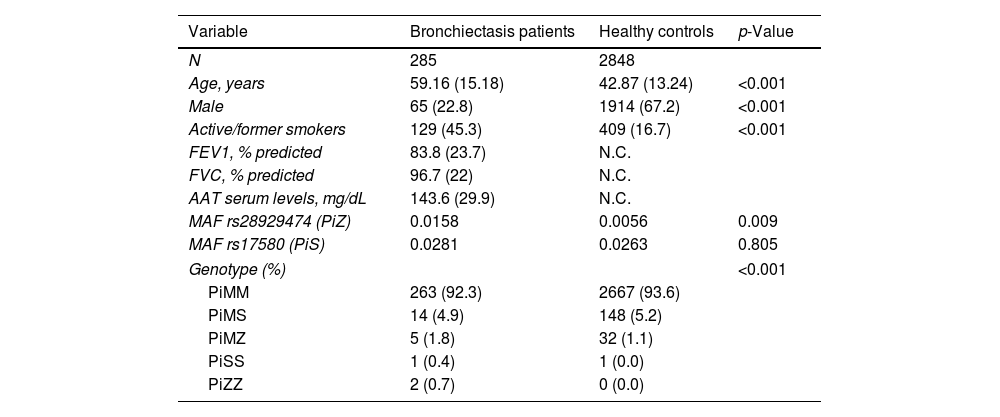
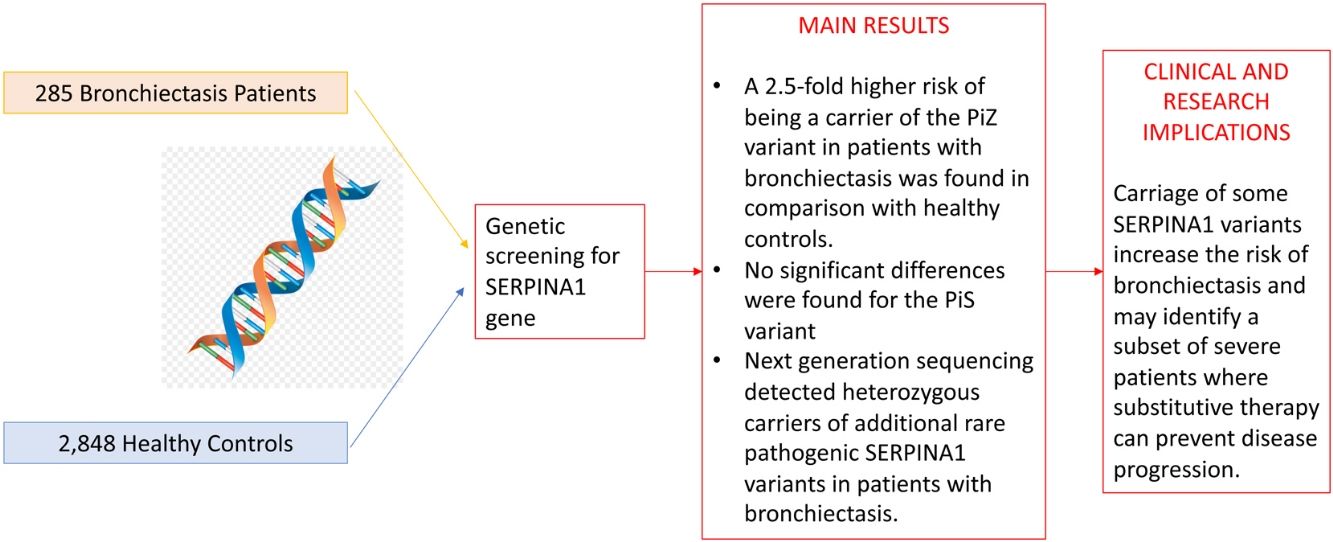
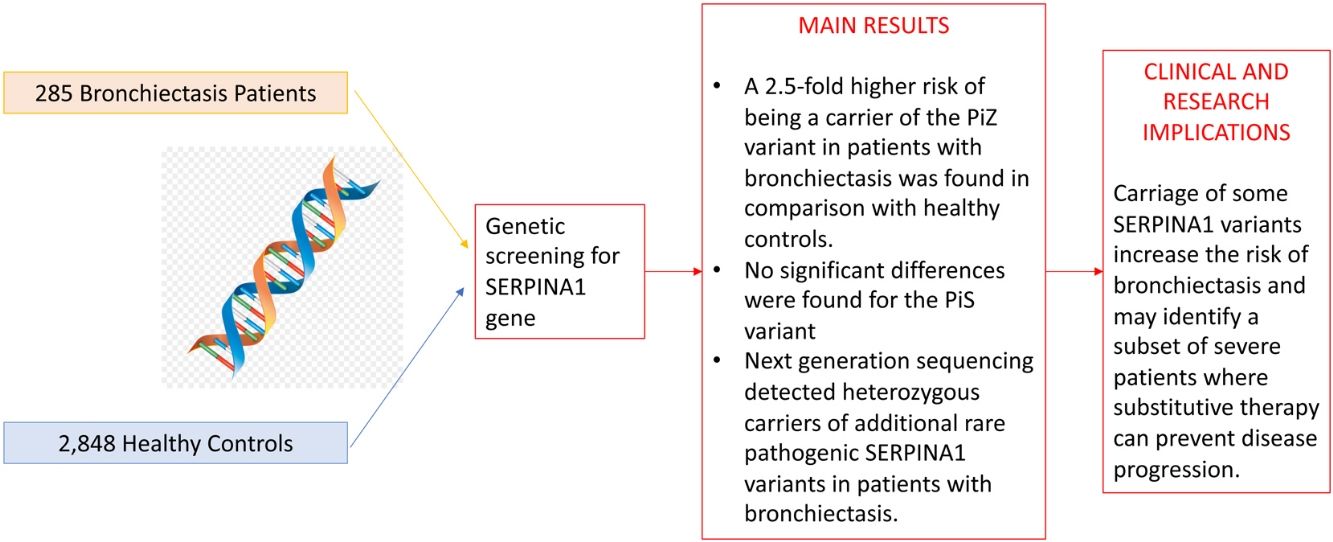
A total of 285 bronchiectasis patients and 2848 healthy blood donors (controls) were enrolled. Bronchiectasis patients were more frequently older female [mean age (SD): 59.16 (15.18) VS. 42.87 (13.24%) years, p-value<0.001, male 65 (22.8%) VS. 1914 (67.2%), p-value<0.001] and current/former-smokers [129 (45.3%) VS. 409 (16.7%), p-value<0.001] (Table 1). The frequency of the PiZ variant was higher in bronchiectasis patients (0.0158 VS. 0.0056, OR: 2.56, 95%CI: 1.19–5.03, p-value: 0.009). No significant differences were found for the PiS variant (OR: 1.07, 95%CI: 0.61–1.75, p-value: 0.805), which is a variant not associated with reduced circulating AAT in heterozygosity.2 Next generation sequencing detected heterozygous carriers of additional rare pathogenic SERPINA1 variants (1 PiDuarte, 1 Q0west, 2 PiMwurzburg, 1 PiI) in patients with bronchiectasis. Among patients, two were homozygous for the PiZ variant and one compound heterozygous for the PiI and PiMwurzburg, SERPINA1 genotypes consistent with the predisposition to develop AATD.
Study cohort and prevalence of PiZ and PiZ SERPINA1 variants in cases.
| Variable | Bronchiectasis patients | Healthy controls | p-Value |
|---|---|---|---|
| N | 285 | 2848 | |
| Age, years | 59.16 (15.18) | 42.87 (13.24) | <0.001 |
| Male | 65 (22.8) | 1914 (67.2) | <0.001 |
| Active/former smokers | 129 (45.3) | 409 (16.7) | <0.001 |
| FEV1, % predicted | 83.8 (23.7) | N.C. | |
| FVC, % predicted | 96.7 (22) | N.C. | |
| AAT serum levels, mg/dL | 143.6 (29.9) | N.C. | |
| MAF rs28929474 (PiZ) | 0.0158 | 0.0056 | 0.009 |
| MAF rs17580 (PiS) | 0.0281 | 0.0263 | 0.805 |
| Genotype (%) | <0.001 | ||
| PiMM | 263 (92.3) | 2667 (93.6) | |
| PiMS | 14 (4.9) | 148 (5.2) | |
| PiMZ | 5 (1.8) | 32 (1.1) | |
| PiSS | 1 (0.4) | 1 (0.0) | |
| PiZZ | 2 (0.7) | 0 (0.0) | |
Data are presented as mean (SD) and n (%).
Legend: MAF: minor allele frequency; FEV1: forced expiratory volume in 1 second; N.C.: not collected; FVC: forced vital capacity; AAT: alpha-1 antitrypsin.
A prevalence of 2.72% for PiZ patients was reported by Cuvelier et al. in bronchiectasis patients in comparison with our 1.58%.4 The epidemiological difference can be explained by the enrolled population (being the prevalence of PiZ higher in Northern Europe) or by the diagnosis of bronchiectasis which was standardized during the past decade.8 Nevertheless, our data are in line with a recent study comparing SERPINA1 genotypes in different chronic respiratory diseases, including bronchiectasis, in Germany and reporting a PiZ prevalence of 1.43%. However, no comparisons with the healthy population are available.9 Notably, five patients were PiMZ in our bronchiectasis cohort. In the absence of external risk factors, such as smoking or respiratory infection, lung manifestations are not frequently developed in these patients. Thus, proteases’ over-reactivity and antiprotease deficiency may have a role in increasing inflammation contributing to bronchiectasis onset and progression.10 Although our experience is strengthened by a detailed genetics analysis of consecutive adults with bronchiectasis, some limitations should be acknowledged. Our findings may not be generalized to other ethnic groups, being limited by the single centre design and the small sample size. Demographic differences between bronchiectasis patients and healthy controls should be acknowledged. In summary, we found a 2.5-fold higher risk of being a carrier of the PiZ variant in patients with bronchiectasis in comparison with healthy controls. Although independent validation in larger multicentre cohorts is still required, these data are consistent with the hypothesis that carriage of some SERPINA1 variants increase the risk of bronchiectasis and may identify a subset of severe patients where substitutive therapy can prevent disease progression.
Authors’ contributionSA, AG, MS, FM, FA, AC, and FB had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. SA, AG, MM, GS, FA, ES, DP, SP, IF, EB, AS, MS, LS, AB, LV and FB contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Funding informationThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interestProf. Aliberti reports consulting fee from Grifols outside the submitted work. Dott. Gramegna reports payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from Grifols outside the submitted work. Prof. Ferrarotti reports payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from Grifols outside the submitted work. Prof. Blasi reports payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from Grifols outside the submitted work.