COPD is a major public health concern, often complicated by cardiovascular comorbidities. Single inhaler triple therapy (SITT) has been proposed as a superior treatment option compared to single inhaler double therapies (SIDT) as LABA/LAMA and LABA/ICS. This systematic review and meta-analysis aim to evaluate the comparative efficacy of these therapies in reducing cardiovascular mortality, major adverse cardiovascular events (MACEs), and all-cause mortality (ACM).
MethodsWe conducted a systematic review and metanalysis including RCT studies comparing SITT with LABA/LAMA or LABA/ICS with mortality as efficacy or safety endpoints. Articles were selected after reviewing PubMed, SCOPUS, Embase, Scielo and clinicaltrials.gov and clinicaltrialsregister.eu from May’24 to Jul’24. Random-effects models were used to estimate the pooled odds ratios (HRs) and 95% confidence intervals (CIs) for cardiovascular mortality, MACEs, and ACM. Heterogeneity and publication bias were assessed using standard statistical methods.
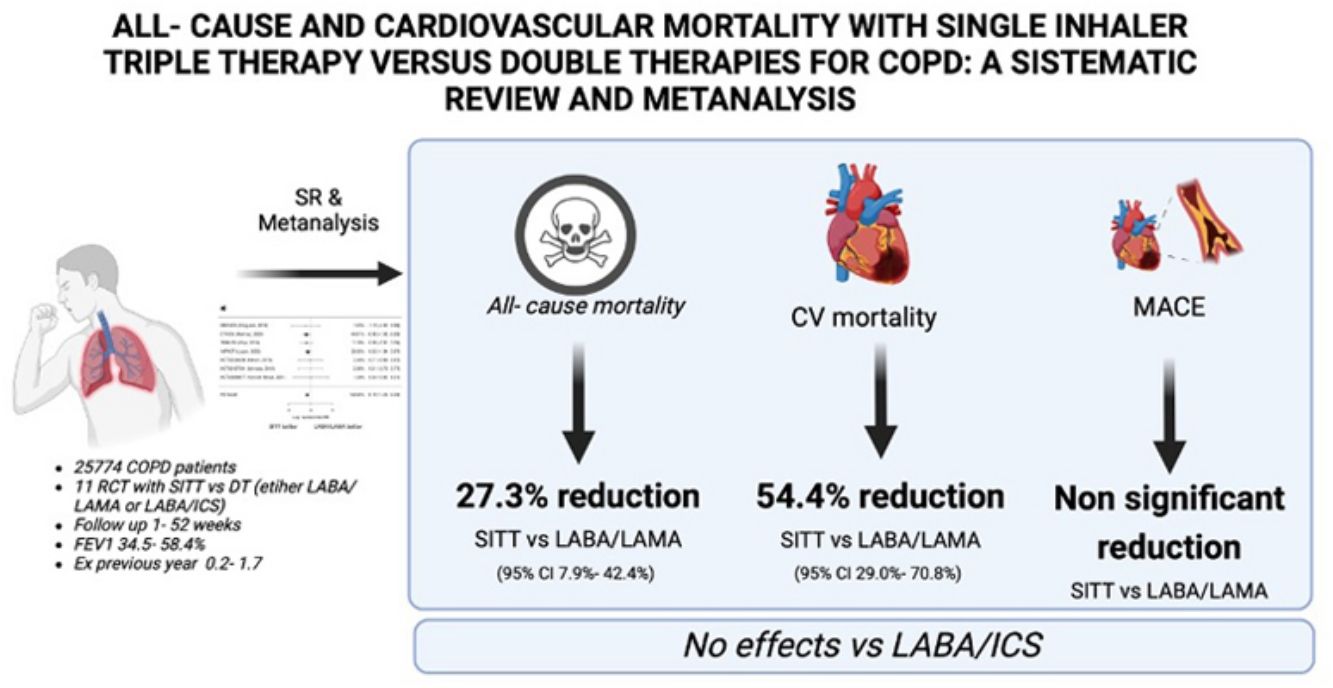
ResultsThe systematic review yielded 568 studies of which 11 were finally included, with 25,774 COPD patients. SITT was superior to LABA/LAMA on ACM (pooled HR 0.727; 95% CI 0.574–0.921, p=0.008) and cardiovascular mortality (pooled HR 0.455; 95% CI 0.292–0.710, p<0.001), with no effect on MACEs. SITT showed no difference versus LABA/ICS on ACM, cardiovascular mortality or MACEs.
ConclusionsSITT significantly reduces cardiovascular and all-cause mortality compared to LABA/LAMA. Compared to LABA/ICS, SITT does not show a significant difference.
PROSPERO IDENTIFIER: CRD42024510253.
Chronic Obstructive Pulmonary Disease (COPD) is one of the most prevalent diseases today and its impact on the population is expected to continue to grow in the coming years.1 According to the World Health Organization (WHO), it is the third leading cause of death worldwide, accounting for 6% of all deaths, only behind coronary heart disease and cerebral vascular disease.2 Moreover, the association between COPD and cardiovascular diseases is known to be strong,3–5 with a third of COPD patients (especially those with moderate COPD) dying from cardiovascular diseases.6 All this joined evidence has made COPD to be considered as a major cardiovascular risk factor.7
The GOLD (Global Obstructive Lung Disease) strategy document identifies two main goals for therapy in COPD: reduce symptom burden and reduce future risk. GOLD acknowledges that there are several pharmacological and non-pharmacological therapies that can be prescribed to patients with COPD that target these two goals.8 One of these interventions is triple therapy (a combination of LAMA – long-acting muscarinic antagonist–, LABA – long-acting beta agonist– and ICS – inhaled corticosteroids), which can improve lung function, improve chronic respiratory symptoms and health related quality of life (HRQoL) and reduce exacerbations.9–15 GOLD recommends SITT (single inhaler triple therapy) for patients in E group (high risk) either as first-line therapy (in patients with high blood eosinophils) or in patients not controlled with LABA/LAMA.
In the latest years two randomized clinical trials (RCT) have shown a reduction in all-cause mortality (ACM) with SITT compared to LABA/LAMA during a 52 week follow up in symptomatic patients with frequent exacerbations.10,11 These findings were subsequently confirmed in two post hoc analysis that comprised most of the recruited participants16,17 with known vital status at week 52. The overall reduction in the ACM in these two trials seemed to be driven by a reduction in the cardiovascular mortality rates among participants. However, there is still a debate whether SITT could reduce mortality in COPD.18,19
In view of the discussion on mortality reduction and cardiovascular consequences of SITT in COPD patients we developed a systematic review and meta-analysis which included RCTs with SITT and a SIDT (single inhaler double therapy, either LABA/ICS or LABA/LAMA) and reported ACM and cardiovascular mortality outcomes.
MethodsStudy DesignThis is a systematic review and metanalysis of randomized clinical trials comparing SITT vs SIDT (either LAMA/LAMA or LABA/ICS) in accordance with PRISMA statement.20 The protocol was registered at PROSPERO under identification number CRD42024510253.
A literature search was performed among databases (PubMed, Embase, Scielo, SCOPUS) as well as in clinicaltrials.gov and clinicaltrialsregister.eu from May 2024 to July 2024. The literature search strategy can be accessed at Supplementary material.
Study PopulationThe study population included RCTs recruiting adult (more than 40 years old) patients with COPD (defined as FEV1/FVC postbronchodilator<0.7), former or current smokers with more than 10 pack-years. The intervention was SITT (LABA/LAMA/ICS fixed dose combinations – FDC) compared to SIDT (either LABA/LAMA FDC or LABA/ICS FDC).
Inclusion and Exclusion CriteriaThe inclusion criteria were RCTs that randomized patients with COPD with at least 1 week of follow-up, included a SITT arm and compared at least a SIDT arm, with assessments of all-cause mortality (ACM), cardiovascular mortality and MACE as efficacy or safety endpoints. Studies should have been published in English, Spanish or Chinese languages. Exclusion criteria were non-RCTs (i.e., observational studies), non-COPD population, not having a SITT or SIDT arm to compare (i.e. open triple arm).
Two reviewers independently checked the relevant RCTs found from literature and databases. RCTs were selected in agreement with the previously mentioned criteria, and any difference in opinion about eligibility was resolved by a third independent reviewer.
OutcomesThe main outcome of this systematic review and metanalysis was to compare ACM between patients on SITT and patients on SIDT (either LABA/LAMA or LABA/ICS). Secondary outcomes included specific cardiovascular mortality and MACES (defined as the occurrence of cardiovascular death, myocardial infarction, acute coronary syndrome, stroke, heart failure and atrial fibrillation).
Quality AssessmentThe methodological quality of included studies was assessed using the Cochrane Risk of Bias (RoB 2).21 The assessment focused on selection bias, performance bias, detection bias, attrition bias, and reporting bias. Studies were categorized as having low, moderate, or high risk of bias.
Statistical AnalysisThe statistical analysis for this meta-analysis was conducted using random-effects models to account for variability both within and between the included studies. For each comparison, the log hazard ratio (HR) and its standard error were calculated for cardiovascular mortality, major adverse cardiovascular events (MACEs), and all-cause mortality. The log hazard ratios were then back transformed to hazard ratios for interpretability.
Heterogeneity among the studies was evaluated using the Q statistic and the I2 statistic. A value of I2 greater than 50% was considered indicative of substantial heterogeneity. Model fit was assessed using the log-likelihood, Akaike Information Criterion (AIC), and Bayesian Information Criterion (BIC).
Publication bias was assessed using the Rosenthal Fail-safe N calculation, which estimates the number of additional studies needed to nullify the observed effect, and funnel plot asymmetry tests, including Kendall's Tau and the regression test for funnel plot asymmetry.
The statistical significance of the results was determined using Z-tests for the log odds ratios, with p-values less than 0.05 considered statistically significant. Confidence intervals (95% CIs) were also calculated to provide a range of values within which the true effect size is expected to lie. All statistical analyses were performed using Jamovi software (version 1.6) with the ‘metafor’ package from R software, which is specifically designed for conducting meta-analyses in R.
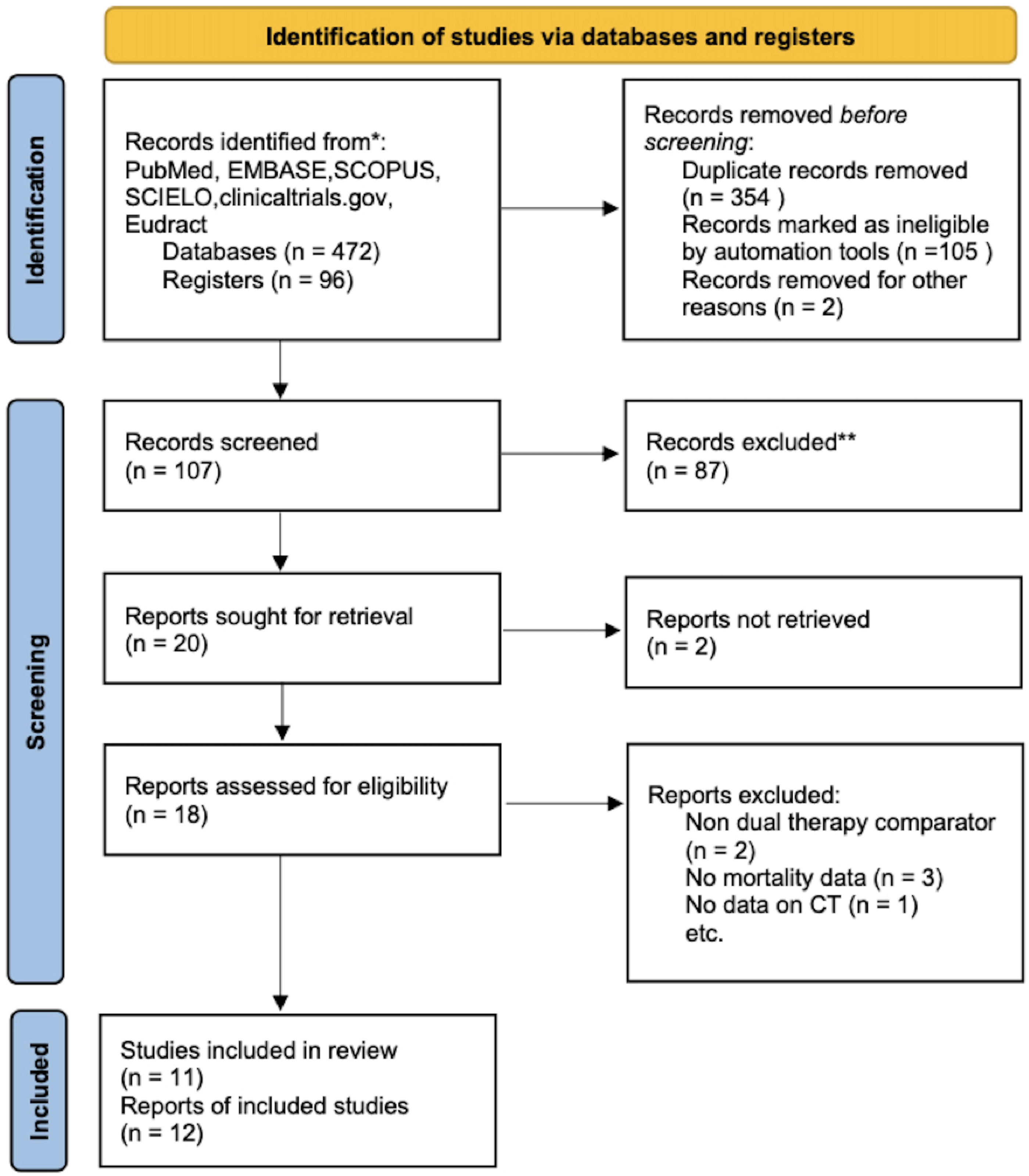
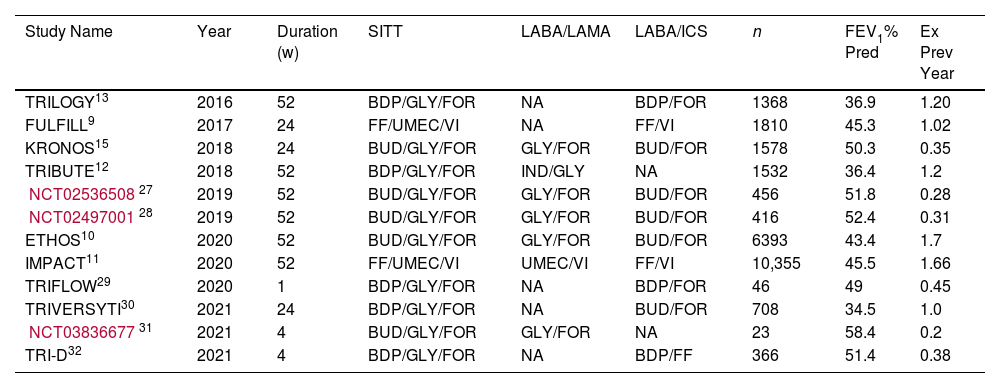
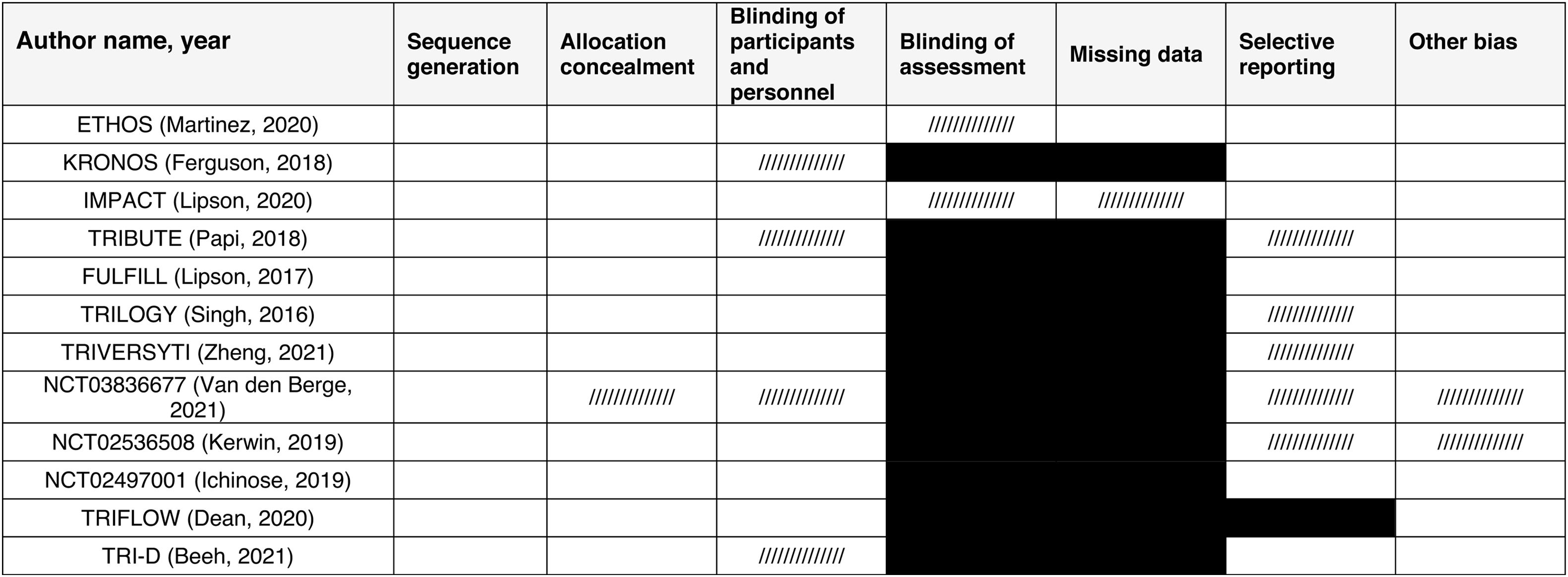
ResultsThe systematic review flow diagram is shown in Fig. 1. Initially, a comprehensive search identified 568 records from various databases. After removing 354 duplicates and excluding 107 records using automation tools and manual checks for eligibility, 107 records were screened. From these, 87 records were excluded based on predefined criteria. Twenty reports were sought for retrieval, but 2 could not be obtained. The remaining 18 reports were assessed for eligibility, resulting in the exclusion of 7 reports for reasons such as non-dual therapy comparators, lack of mortality data, and absence of combination therapy data. Ultimately, 11 studies, reported across 12 publications, were included in the final review and analysis. A summary of the studies included can be found in Table 1. The total number of patients included in this meta-analysis is 25,774. The methodological quality of included studies RoB 2 table is presented in Table 2.
Studies Included in the Systematic Review and Baseline Patient Characteristics.
| Study Name | Year | Duration (w) | SITT | LABA/LAMA | LABA/ICS | n | FEV1% Pred | Ex Prev Year |
|---|---|---|---|---|---|---|---|---|
| TRILOGY13 | 2016 | 52 | BDP/GLY/FOR | NA | BDP/FOR | 1368 | 36.9 | 1.20 |
| FULFILL9 | 2017 | 24 | FF/UMEC/VI | NA | FF/VI | 1810 | 45.3 | 1.02 |
| KRONOS15 | 2018 | 24 | BUD/GLY/FOR | GLY/FOR | BUD/FOR | 1578 | 50.3 | 0.35 |
| TRIBUTE12 | 2018 | 52 | BDP/GLY/FOR | IND/GLY | NA | 1532 | 36.4 | 1.2 |
| NCT0253650827 | 2019 | 52 | BUD/GLY/FOR | GLY/FOR | BUD/FOR | 456 | 51.8 | 0.28 |
| NCT0249700128 | 2019 | 52 | BUD/GLY/FOR | GLY/FOR | BUD/FOR | 416 | 52.4 | 0.31 |
| ETHOS10 | 2020 | 52 | BUD/GLY/FOR | GLY/FOR | BUD/FOR | 6393 | 43.4 | 1.7 |
| IMPACT11 | 2020 | 52 | FF/UMEC/VI | UMEC/VI | FF/VI | 10,355 | 45.5 | 1.66 |
| TRIFLOW29 | 2020 | 1 | BDP/GLY/FOR | NA | BDP/FOR | 46 | 49 | 0.45 |
| TRIVERSYTI30 | 2021 | 24 | BDP/GLY/FOR | NA | BUD/FOR | 708 | 34.5 | 1.0 |
| NCT0383667731 | 2021 | 4 | BUD/GLY/FOR | GLY/FOR | NA | 23 | 58.4 | 0.2 |
| TRI-D32 | 2021 | 4 | BDP/GLY/FOR | NA | BDP/FF | 366 | 51.4 | 0.38 |
SITT: single inhaler triple therapy; LABA: long-acting beta agonist; LAMA: long-acting muscarinic antagonist; ICS: inhaled corticosteroid; BDP: beclomethasone dipropionate; GLY: glicopirronium; FOR: formoterol; FF: fluticasone furoate; UMEC: umeclidinium; VI: vilanterol; BUD: budesonide. FEV1% pred: forced respiratory volume in the first second as a percentage of predicted; Ex: moderate & severe exacerbations in previous year.
Risk of Bias (RoB 2) Assessment Among the Included Studies.
a Other bias refers to bias due to problems not covered elsewhere in the table (e.g. the study had a potential source of bias related to the specific study design used, or there is insufficient information to assess whether an important risk of bias exists, or insufficient rationale or evidence that an identified problem will introduce bias).
White: low risk of bias; pattern ///////: unclear risk of bias; black: high risk of bias.
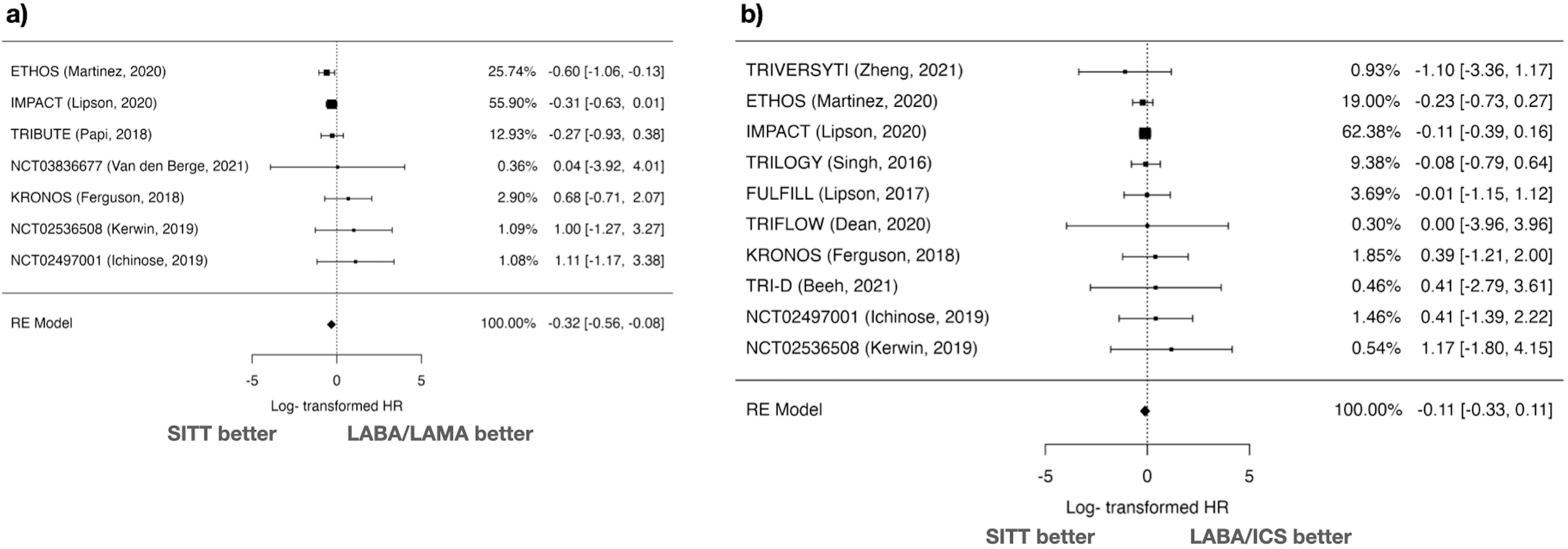
The comparison of ACM between SITT and LABA/LAMA included 7 studies. The log HR was −0.319 with a standard error of 0.121, resulting in a statistically significant Z value of −2.64 (p=0.008) (Fig. 2). The 95% CI ranged from −0.556 to −0.082. The back-transformed HR was 0.727 (95% CI: 0.574–0.921). There was no significant heterogeneity (I2=0%, Q=6.175, p=0.404), and the Fail-safe N was 0 (p=0.178). Funnel plot analyses showed no significant asymmetry (Kendall's Tau=0.429, p=0.239; Regression Test Z=1.791, p=0.073) (Supplementary Fig. 1).
In the comparison of ACM between SITT and LABA/ICS, 10 studies were analyzed. The log HR was −0.111 with a standard error of 0.111, resulting in a Z value of −0.993 (p=0.321) (Fig. 2). The 95% CI ranged from −0.329 to 0.108. The back-transformed HR was 0.895 (95% CI: 0.720–1.114). There was no significant heterogeneity (I2=0%, Q=2.498, p=0.981), and the Fail-safe N was 0 (p=0.384). Funnel plot analyses showed no significant asymmetry (Kendall's Tau=0.244, p=0.381; Regression Test Z=0.604, p=0.546) (Supplementary Fig. 1).
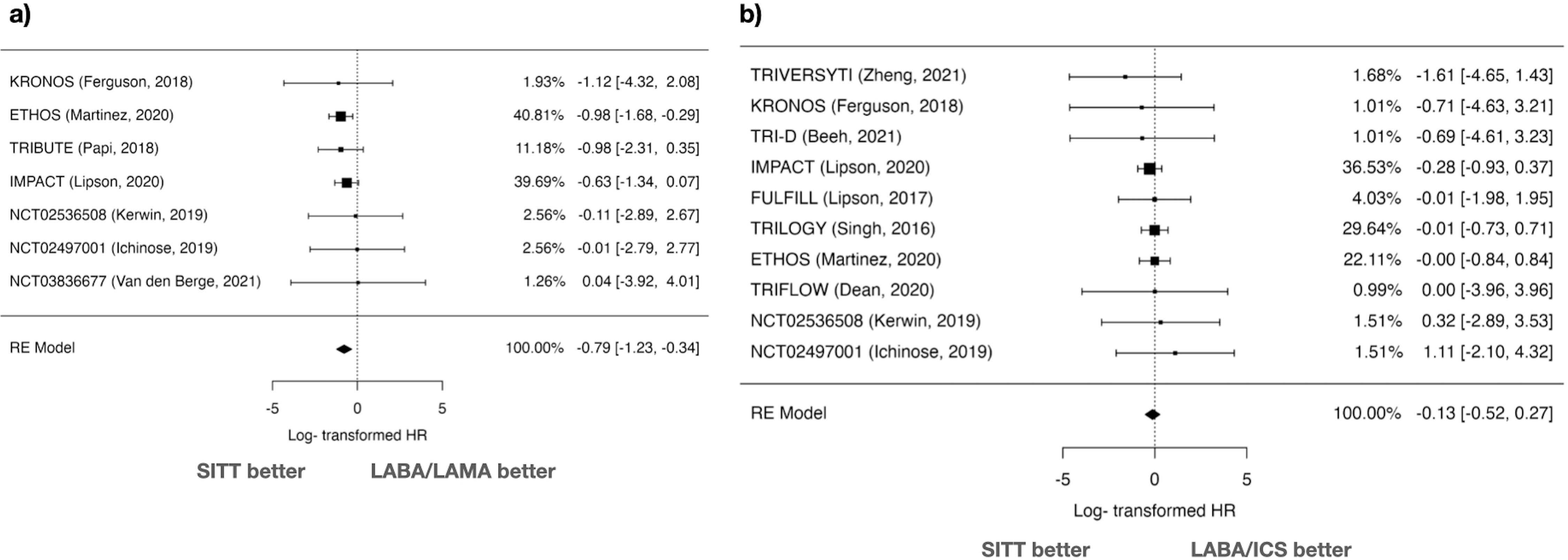
Cardiovascular Mortality AnalysisIn the comparison of SITT versus LABA/LAMA for cardiovascular mortality, the random-effects model analysis included 7 studies. The log HR was −0.787 with a standard error of 0.227, yielding a statistically significant Z value of −3.47 (p<0.001) (Fig. 3). Transforming the log HR to the HR, the result was 0.455 (95% CI: 0.292–0.710), suggesting a significant reduction in cardiovascular mortality with SITT.
Heterogeneity statistics showed no significant heterogeneity (I2=0%, Q=1.308, p=0.971). Publication bias was assessed using the Rosenthal Fail-safe N, yielding a value of 10,000 (p=0.006), and no significant asymmetry was detected in funnel plot analyses (Kendall's Tau=0.238, p=0.562; Regression Test Z=0.542, p=0.588) (Supplementary Fig. 2).
The comparison between SITT and LABA/ICS for cardiovascular mortality involved 10 studies. The log HR was −0.125 with a standard error of 0.201, resulting in a Z value of −0.623 (p=0.533) (Fig. 3). The 95% CI ranged from −0.519 to 0.269, indicating no significant difference. The back-transformed HR was 0.882 (95% CI: 0.595–1.308). There was no significant heterogeneity (I2=0%, Q=2.119, p=0.989), and the Fail-safe N was 0 (p=0.290). Funnel plot analyses showed no significant asymmetry (Kendall's Tau=−0.156, p=0.601; Regression Test Z=−0.100, p=0.920) (Supplementary Fig. 2).
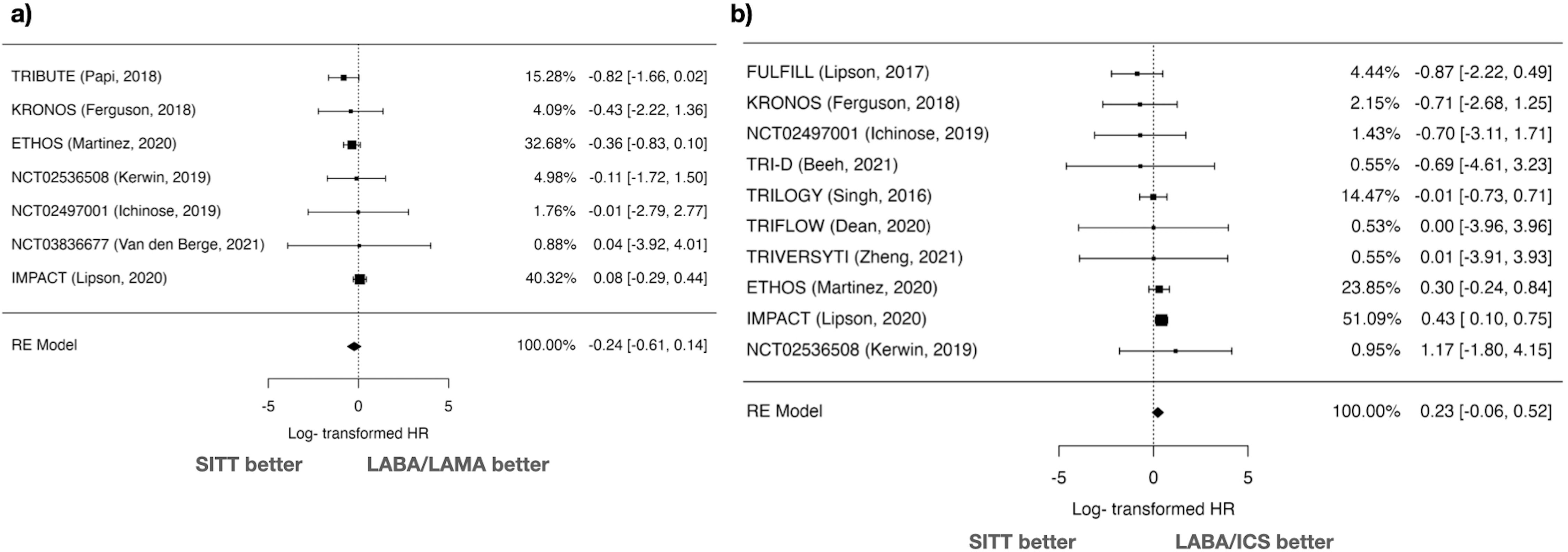
MACEs AnalysisFor MACEs, 7 studies comparing SITT vs LABA/LAMA were analyzed using a random-effects model. The log HR was −0.235 with a standard error of 0.191, resulting in a Z value of −1.23 (p=0.219) (Fig. 4). The 95% CI ranged from −0.610 to 0.139, indicating no significant difference between SITT and LABA/LAMA. The HR was 0.790 (95% CI: 0.543–1.150). Heterogeneity was low (I2=24.17%, Q=4.828, p=0.566), and the Fail-safe N was 0 (p=0.086). Funnel plot asymmetry tests showed no significant publication bias (Kendall's Tau=0.143, p=0.773; Regression Test Z=−0.281, p=0.779) (Supplementary Fig. 3).
Regarding SITT vs LABA/ICS, 10 studies were included. The log HR was 0.231 with a standard error of 0.148, resulting in a Z value of 1.56 (p=0.119) (Fig. 4). The 95% CI ranged from −0.059 to 0.521, indicating no significant difference. The HR was 1.260 (95% CI: 0.942–1.684). Heterogeneity was minimal (I2=5.83%, Q=6.360, p=0.703), and the Fail-safe N was 0 (p=0.316). Publication bias tests indicated no significant asymmetry (Kendall's Tau=0.022, p=1.000; Regression Test Z=−1.481, p=0.139) (Supplementary Fig. 3).
DiscussionIn this systematic review and metanalysis involving more than 25,000 COPD patients, SITT was associated with a significant reduction in all-cause mortality compared to LABA/LAMA, but not compared to LABA/ICS. This suggests that the inclusion of inhaled corticosteroids in combination therapies may play a role in enhancing patient survival. The results also indicate that SITT could be particularly beneficial for patients who are not adequately managed with LABA/LAMA alone.
Our results demonstrate that SITT significantly reduces cardiovascular mortality when compared to LABA/LAMA. This finding is robust, with a substantial reduction in HR and no significant heterogeneity or publication bias detected. The lack of benefit seen in the comparison with LABA/ICS highlights the importance of the specific components of combination therapy in managing cardiovascular risk in patients with COPD. The potential mechanisms of this benefit are currently under investigation, but the reduction of exacerbations, reduction of inflammation at the pulmonary and systemic level, improvement of pulmonary hyperinflation and improvements in tissue hypoxaemia may explain the findings of this meta-analysis.4
The analyses did not show significant differences in MACEs between SITT and either LABA/LAMA or LABA/ICS. This suggests that while SITT may be effective in reducing mortality, it does not have a clear advantage in preventing MACEs. This could have happened due to either differences in MACE definition or the event adjudication among the studies. The effect not seen in MACEs could be explained by different definitions of this outcome or the rates of these events across the different studies included in this metanalysis. Besides that, some recent population- based analysis suggested that SITT could increase MACEs compared to LABA/ICS.22 The variability in outcomes highlights the complexity of cardiovascular risk management in this patient population and underscores the need for personalized treatment strategies.
The primary limitation of this meta-analysis is the relatively small number of included studies, which may affect the power and generalizability of the findings and the fact that the included studies followed up patients at best for 1 year. However, the total number of patients included in this meta-analysis is above 25,000, and most of these studies have less than 10 years since they were published. Additionally, there is a risk of heterogeneity not detected by statistical tests due to variations in study design, treatment duration (some of the studies had less than one month of follow up and other had up to 52 weeks follow up), patient populations (regarding severity, ethnicity and current smoking proportions), and outcome definitions. For example, the design of the studies regarding the way ICS component was stopped is different across the included studies. However, although there are clear differences between the studies included, the heterogeneity was low across the studies and there was no significant publication bias.
Another limitation of this metanalysis could be the differences in previous exacerbation history, and blood eosinophil counts at baseline, which could have influenced the results. Furthermore, in several included studies there was no event adjudication committee, giving rise to the possibility that some of the reported events were not fully accurate, specially those regarding the cause of mortality and MACEs. However, the main RCTs included in this analysis did report a specific event-adjudication committee16,17 and therefore we can be confident that the results are robust enough.
We included only SITT studies and not multiple inhaler triple therapy (MITT) studies in this analysis because there is some evidence that suggests that SITT improves mortality and exacerbations compared to MITT due to an increased adherence.23–25 It seems that MITT could also have an impact on ACM, but whether this could apply to cardiovascular death is not known.26 The exclusion of MITT studies in this metanalysis limits the generalizability of the finding to real-world clinical practice.
SITT is recommended by GOLD to patients at risk for future events or those not controlled on LABA/LAMA. However, our analysis included studies involving patients at risk for future outcomes (i.e.: exacerbations) as well as patients without future risk. Future studies should specifically study the effect of SITT on all-cause mortality and cardiovascular-specific mortality among patients who do not have frequent exacerbations.
ConclusionsThis meta-analysis provides evidence supporting the use of SITT in reducing cardiovascular and all-cause mortality in patients with COPD. However, its impact on major adverse cardiovascular events remains unclear, highlighting the need for further research. The findings underscore the importance of non- respiratory outcomes of inhaled therapies and suggest that SITT could be a valuable option in the therapeutic arsenal for managing COPD and cardiovascular comorbidities.
FundingThis research did not receive any funding from private or public sources.
Conflicts of InterestAH reports non-financial support from Bial and Chiesi. CHS has nothing to disclosure. ARL has nothing to disclosure. LAM has nothing to disclosure. AML has nothing to disclosure. DRN has nothing to disclosure. AJA has nothing to disclosure. AAL has nothing to disclosure. LCMM has nothing to disclosure. ESA has nothing to disclosure. PJRP reports personal fees and non-financial support from GSK, non-financial support from Novartis AG, non-financial support from Boehringer Ingelheim, non-financial support from Chiesi, grants, personal fees and non-financial support from Laboratorios Menarini, personal fees from Esteve, outside the submitted work. BAN reports grants and personal fees from GSK, grants, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, personal fees and non-financial support from Chiesi, grants, personal fees and non-financial support from Laboratorios Menarini, personal fees from Bial, personal fees from Zambon, personal fees from Gilead, personal fees from MSD, and personal fees from Sanofi, outside the submitted work
Data AvailabilityThe datasets generated and analyzed during the current study are available from the corresponding author on reasonable request. Additionally, data supporting the findings of this study can be found in the cited references and Supplementary materials of the included studies.
The authors want to acknowledge the support from the Research Unit at their hospital for helping to perform this review.