Multidrug-resistant tuberculosis (MDR-TB) represents a global challenge. In 2018, 186,772 new cases of MDR-TB or TB resistant to rifampicin (RR-TB) were notified globally, resulting in an estimated incidence of 484,000 (95% confidence interval 417,000–556,000).1 Mathematical modelling studies estimate the number of MDR-TB in children as between 25,000 and 32,000 annually, although it is suspected that <5% are identified and even less are correctly treated.2
Treatment outcomes for MDR-TB remain suboptimal. In the last 40 years only two new drugs for TB treatment have been licensed (bedaquiline and delamanid).3 However, the World Health Organization (WHO) has recently updated its guidelines on MDR-TB treatment, as well as the SEPAR guideline updated in 2017.4,5 They propose new drug combination regimens which exclude the second-line injectable agents and include bedaquiline, delamanid and other repurposed drugs such as linezolid and clofazimine in order to optimize safety and efficacy.4 Bedaquiline is considered highly effective and is recommended in all MDR-TB regimens for adults and children>6 years; however, it is expensive and not currently available in many European countries such as Spain. Bedaquiline dosing and safety studies are yet to be done in young children (<6 years). Linezolid is also recommended as a core agent with good activity against M. tuberculosis including extensively drug-resistant (XDR) strains. Fluoroquinolones (levofloxacin and moxifloxacin) complete the group of the most effective agents (Group A).4 The major advantage is that they are all-oral regimens, increasing treatment feasibility and reducing severe adverse events such as ototoxicity and nephrotoxicity caused by the aminoglycosides. However, these new drug regimens are not exempt from adverse events and data on safety and efficacy in children are scarce.2 We present a case of probable cycloserine-associated behavioural disturbance with neuromuscular symptoms, as well as intracranial hypertension secondary to levofloxacin in a child with MDR-TB.
A previously healthy, not BCG-vaccinated, Spanish six-year-old girl was diagnosed with tuberculosis (TB) following active household contact tracing. Her father, diagnosed with infectious MDR pulmonary TB, was the index case.
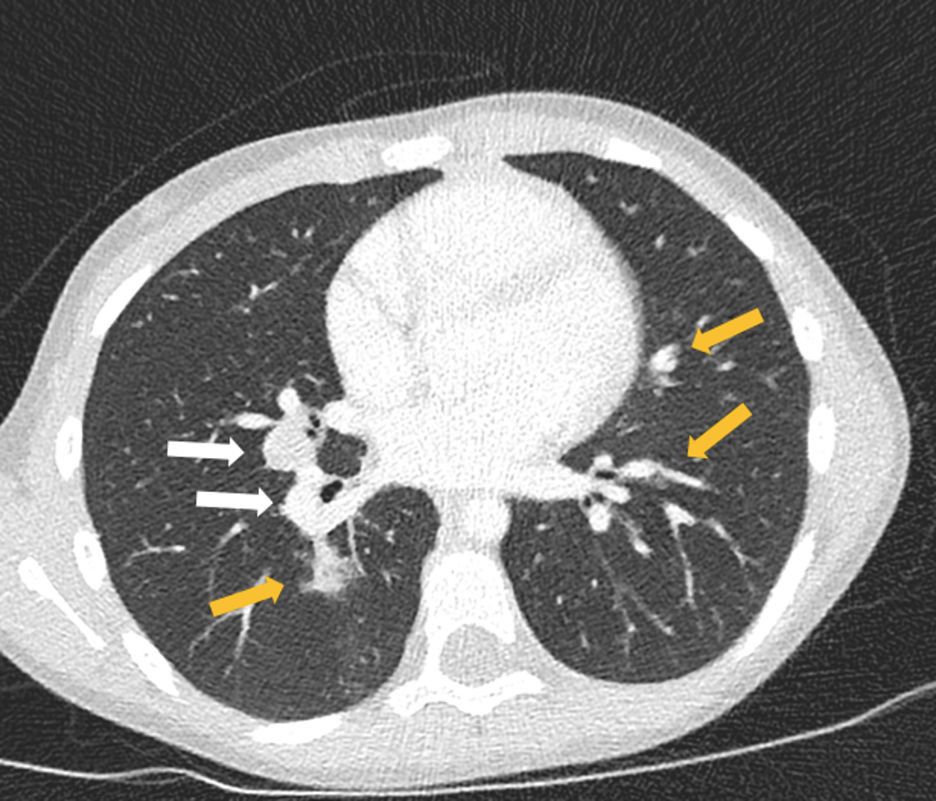
At diagnosis she was asymptomatic, with normal physical examination and a positive tuberculin skin test (induration of 13mm). Left hilar enlarged lymph nodes were suspected on her chest-X-ray and bilateral enlarged mediastinal and hilar lymph nodes with bilateral lung nodules were detected on chest computed tomography (CT) (Fig. 1). Serial gastric aspirate specimens for acid-fast bacilli smear microscopy, PCR and mycobacterial culture were negative in the patient; however, M. tuberculosis cultures were positive in both her father and four-year old brother, with isolates showing resistance to isoniazid, rifampicin, pyrazinamide, ethionamide and streptomycin. These findings were sufficient for the diagnosis of MDR pulmonary TB, reserving the bronchoscopy in the event that the patient did not have a known TB contact or antimicrobial sensitivity pattern. Therefore, an injectable-free treatment regimen was initiated with levofloxacin (18.5mg/kg/day), linezolid (14mg/kg/day), clofazimine (100mg every second day), cycloserine (18.5mg/kg/day) and ethambutol (18.5mg/kg/day).
Three weeks later, she was admitted to hospital due to vomiting and refusal to take MDR-TB drugs. On admission she presented with right-sided torticollis and an adjustment disorder with mixed disturbance of emotions and conduct including insomnia and an anxiety crisis. Six days later, the patient also developed right peripheral facial palsy. A cranial and cervical spine CT scan did not show any abnormal findings such as an intracranial tuberculoma or cervical spine TB. Complete blood count (CBC), renal, hepatic and mineral panel and thyroid function tests were normal. Rheumatoid factor and anti-nuclear antibodies were negative. Cycloserine was considered as likely responsible for these clinical findings and was discontinued: neuro-psychiatric symptoms progressively disappeared. Delamanid (4mg/kg/day) was added to the treatment regimen to replace cycloserine.
Two weeks later (5 weeks after treatment initiation), bilateral optic disc swelling was detected after performing a routine ophthalmological toxicity examination. She was asymptomatic at this time and a brain magnetic resonance imaging showed a papillary protrusion and posterior flattening of both eyes (indirect signs of intracranial hypertension) without any other abnormal radiological findings. Cytological and biochemical analysis of the cerebrospinal fluid were normal and culture was negative. An increased intracranial pressure (ICP) (34cm H2O) confirmed the diagnosis of intracranial hypertension. Levofloxacin was considered the likely cause and was discontinued. To manage the raised ICP, acetazolamide was added, causing metabolic acidosis, which was treated with oral bicarbonate. Thereafter her clinical resolution was good with a progressive decrease of ICP and after six weeks the fundus examination returned to normal.
At present, she is in the 8th month of treatment and medications including linezolid, clofazimine, delamanid, and ethambutol are well tolerated. She remains asymptomatic presenting an adequate weight gain. Serial chest X-ray are normal and a chest CT will be scheduled at the end of treatment at 15 months from treatment initiation. Clinical follow up including CBC, electrocardiogram and ophthalmologic exam is performed on a monthly basis.
This case report shows the challenges of managing MDR-TB in children. The clinical evolution of this case with several adverse events related to the MDR-TB treatment, highlights the potential risk of adverse effects caused by these new drug combinations and the concern that treatment interruption may compromise treatment efficacy.
Initially, the patient presented with behavioural disturbance after three weeks of MDR-TB therapy. The association of cycloserine with depression, psychosis and neuropathy is well established.3 Cycloserine-associated neuro-psychiatric effects are likely due to its binding to N-methyl-d-aspartate receptors and it has been demonstrated that these adverse events are related to high drug concentrations in serum. This has led to investigate the therapeutic use of cycloserine at lower doses for psychiatric disorders.6 Therapeutic drug monitoring is used to individualize treatment doses and might help to prevent toxicities caused by high drug concentrations; however, it was not performed in this case as it was not locally available. Moreover, based on the presence of right-sided torticollis and lower motor neuron facial palsy, a space-occupying lesion was also ruled out. We finally attributed these neuro-psychiatric symptoms to cycloserine, as they progressively disappeared after the withdrawal of the drug.
The association between fluoroquinolones and intracranial hypertension has been previously described, although it is rare, and the pathogenic mechanism is not completely understood.7 Few case reports have shown specific association of levofloxacin-induced intracranial hypertension between 5 days and 3 months after the start of the treatment.8–10 We decided to discontinue levofloxacin and improvement of her condition was observed in a short period of time.
Fluoroquinolones are a key component for MDR-TB treatment. When contraindicated, the therapeutic strategy should be planned as extensively drug-resistant tuberculosis. Therefore, she eventually continued with a “non-optimal” regimen with good radiological response.
Despite the improvement in therapeutic strategies for MDR-TB, this case points out the need to keep searching for the most effective and least toxic drug regimen(s) for MDR-TB in children and in adults.