CURRENT SITUATION AND LONG-TERM CONSEQUENCES OF COVID-19 INFECTION
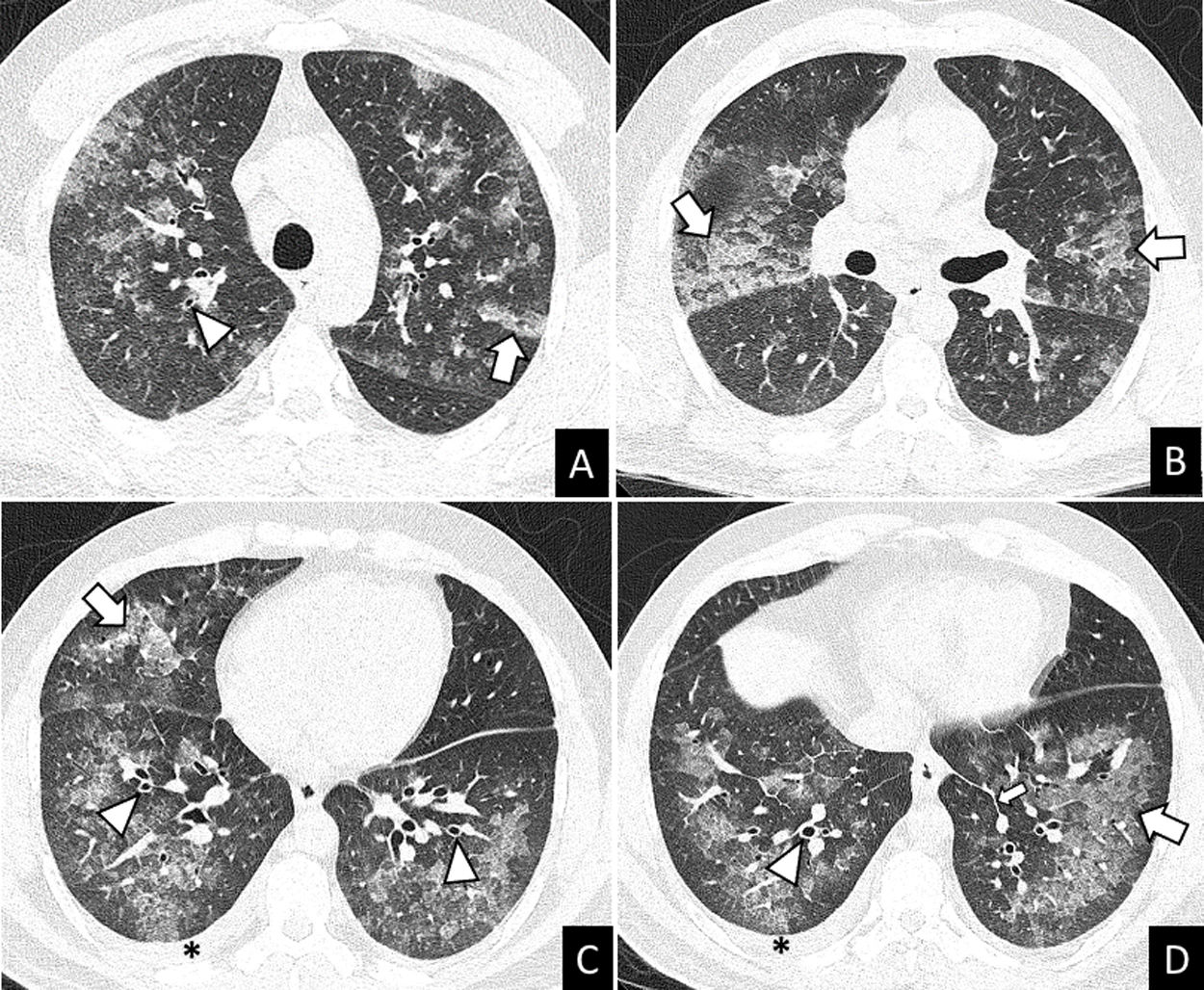
More infoA previously healthy 37-year-old male was admitted to hospital after two days of mild fever, dyspnea, wheezing, oppressive chest pain, dry cough, palpitations and arthromyalgia that started just 8-10h after the second dose of Pfizer-BioNTech COVID-19 vaccine. The chest X-ray showed multilobar and coalescent alveolar opacities. Serial negative nasopharyngeal swab reverse transcriptase-polymerase chain reaction (RT-PCR) tests ruled out SARS-CoV-2 infection. Unenhanced chest CT performed a few hours after admission revealed bilateral ground glass opacities (GGO) and consolidation with interlobular septal thickening, peribronchial cuffing, and mild bilateral pleural effusion (Fig. 1). The analysis of bronchoalveolar lavage (BAL) allowed to ruled out infection and showed differential cell count of 60.3% eosinophils. These findings suggested an acute eosinophilic pneumonia (AEP). Blood eosinophils gradually increased during the hospitalization (0.7–4.13109/L). Due to the spontaneous clinical improvement, the patient was discharged five days after admission.
AEP is an eosinophilic lung disease characterized by the sudden onset of respiratory symptoms that often occur in healthy young individuals.1,2 The characteristic histopathological finding of AEP is eosinophilic infiltration in the alveolar and interstitial spaces. The mechanism for the overproduction and accumulation of eosinophils is unknown, although cytokines (interleukin 5), may play an important role.
AEP can be diagnosed with the modified Philit criteria4: (1) acute respiratory illness ≤1 month of duration; (2) typical radiological features; (3) pulmonary eosinophilia of more than 25% eosinophils in BAL fluid (increased percentages of lymphocytes and neutrophils could be found) or eosinophilic pneumonia on lung biopsy and (4) absence of other specific pulmonary eosinophilic diseases. Imaging findings include patchy or diffuse pulmonary opacities with interlobular septal thickening and mild to moderate pleural effusion.3 Lung biopsy is not usually required. Individuals with AEP can present neutrophilic leukocytosis without eosinophilia in peripheral blood in early stages of the disease. In many cases, the eosinophil count in peripheral blood increases during the following days.2 Treatment of AEP consists of systemic corticosteroids after ruling out an infectious cause of pulmonary eosinophilia. In some cases, AEP can improve spontaniously.1
In our patient, the diagnosis of AEP was established based on the radiological findings, the absence of a concurrent infection, and the increased eosinophil count in the BAL fluid. Given the sudden and rapid onset of patient's symptoms within hours of the second dose of the vaccine, we believe that mRNA-based COVID-19 vaccine triggered the eosinophilic response, similar to some other viral infections or drugs.
Previous studies have reported AEP following the administration of vaccines, including influenza vaccination5 and the 23-valent-pneumococcal-polysaccharide vaccine.6 A recently published case report describes AEP following COVID-19 vaccination with adenovirus vaccine AZD1222.7 Interstitial lung diseases associated to COVID-19 mRNA vaccination have also been reported.8 Here, we report the first case of AEP following mRNA COVID-19 vaccination confirmed by BAL. Definitive proof of the direct link between this condition and the mRNA based COVID-19 vaccine is still uncertain and further investigation is needed to prove causation.
Authors’ contributionThe above listed authors attest that they made substantial contributions to the (1) conception and design, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be submitted for revision. All authors had full access to all the data in the study and accept responsibility for the submission of this work.
None.