Gordonia species are gram positive, catalase positive, partially acid-fast aerobic actinomycetes that belong to the genus Gordonia.1 Most of the species within Gordonia genus, have been isolated from environmental sources such as soil, farm animals and water.1–3Gordonia bronchialis (G. bronchialis) was originally isolated from human clinical specimens.2,5 Although they rarely cause infection in humans,1,4 it is believed that their transmission is primarily through the respiratory inhalation of bacteria.2 A number of case reports have been published in the medical literature, but to our knowledge this is the first report of pneumonia caused by G. bronchialis.
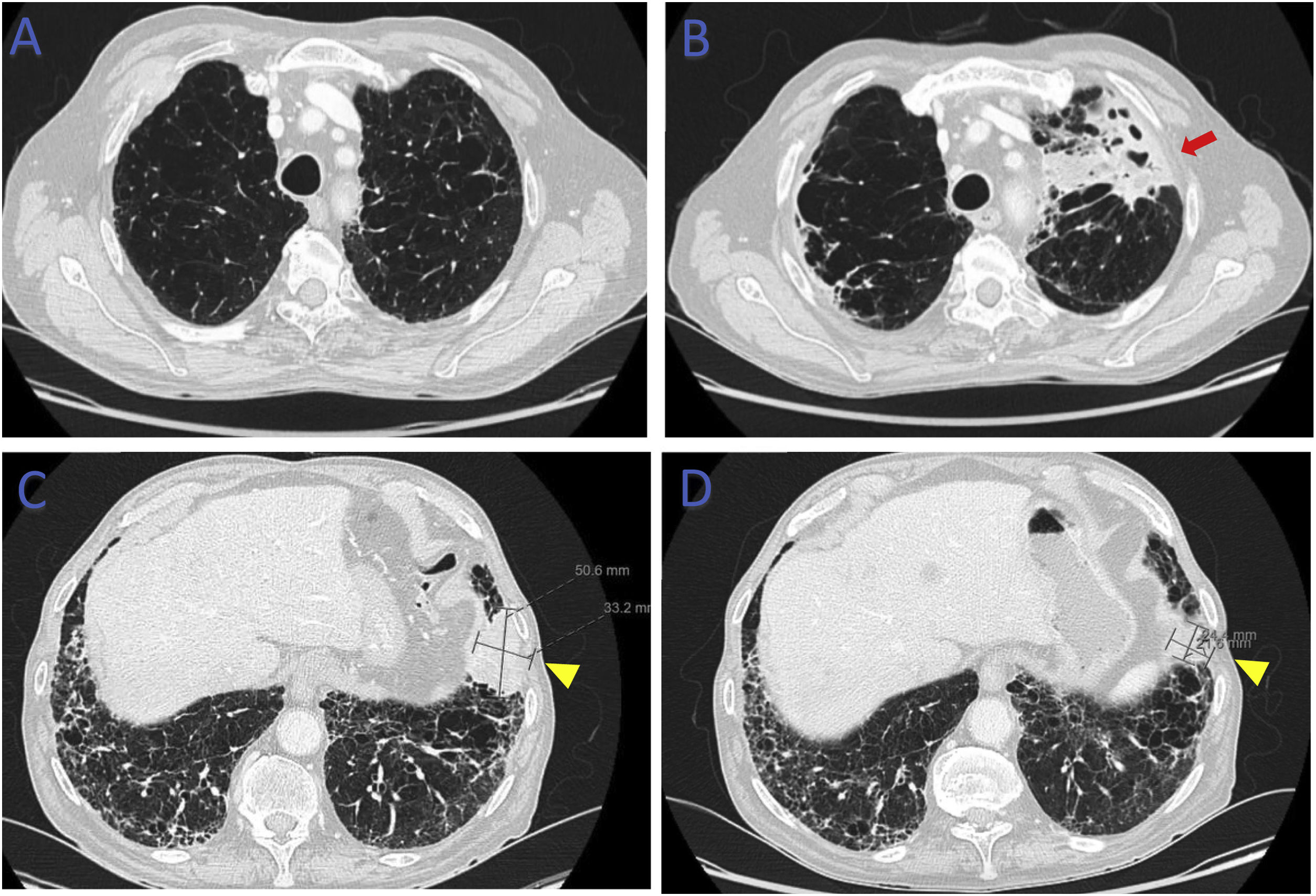
78-Year-old male, smoker, with multiple comorbidities, including combined pulmonary fibrosis and emphysema (CPFE) and stage 4 small cell lung carcinoma (SCLC) under treatment with carboplatine/etoposide (CT), presented at the Oncology follow-up visit with complaints of asthenia and increased bronchorrhea. The patient did not present fever or respiratory insufficiency, however there was an increase in inflammatory markers. The follow-up chest computed tomography (CT) scan showed a positive response to CTx (decrease in size of carcinoma), but a new opacification on the left superior lung lobe with areas of mucoid impactation and cavitation (Fig. 1).
(B) Computed tomography of the chest (lung window) showing opacification on the left superior lung lobe (red arrow) not previously present (A). Computed tomography of the chest (lung window) showing lung carcinoma in the left inferior lobe (yellow arrowhead) before (C) and at the time (D) of reported symptoms.
To study the aetiology of this lesion, flexible bronchoscopy was performed. The patient started empirical antimicrobial therapy with amoxicillin–clavulanic acid 875mg/125mg every 12h for 7 days and CTx was suspended. Routine bronchoalveolar lavage (BAL) bacterial [cut-off ≥104 colony forming units (CFU)/mL] and fungal cultures were negative. Detection of Mycobacterium tuberculosis complex by real-time polymerase chain reaction (RT-PCR) using Xpert® MTB/RIF Ultra (Cepheid) was also negative. Mycobacteriological culture on Löwenstein-Jensen medium revealed the presence of pigmented, dry and raised colonies, later identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) using VITEK® MS (bioMérieux) as G. bronchialis. Identification was confirmed by 16S rRNA gene sequencing. As there are no standardized laboratory methods for aerobic actinomycetes other than Nocardia species, antimicrobial susceptibility testing (AST) was not performed. Despite a positive clinical (resolution of bronchorrhea) and analytical response to empirical antibiotics, 2 months after, the overall status worsened significantly and it was decided best supportive care.
G. bronchialis has only recently been associated with infection in humans. In the past, G. bronchialis infections were probably underdiagnosed due not only to the specific biological features of aerobic actinomycetes, but also to the difficulties in achieving species-level identification using conventional laboratory methods. In fact, due to its fastidious and slow-growing nature, G. bronchialis is generally unable to grow in routine bacterial cultures, which was exactly what happened in our case, where the bacterial BAL culture was ruled out as normal respiratory microflora. With the improved accuracy of laboratory identification methods, it is likely that the number of identified infections due to G. bronchialis will rise. To our knowledge, this is the first case report of G. bronchialis pneumonia. Currently, there are no standardized treatment guidelines for Gordonia infections. The choice and duration of antimicrobial therapy are often empirical, but should be adjusted based on patient individual response.
ParticipationAll authors confirm that they have participated actively in the preparation of this article.
FundingNo funding to declare.
Conflict of interestNone to be declare.