Over the last 2 decades, calls for transparency in medical research have changed the way clinical trial results are reported. The requirement to register trials with medications and to publish the results have contributed greatly to this transparency. However, society now demands that the anonymized individual data of clinical trial participants should be freely accessible,1 on the grounds that this information belongs to the public domain. This appears obvious for publicly funded trials, but is equally so for privately funded studies, and this is how for-profit (https://clinicalstudydatarequest.com/) or non-for-profit (http://www.gatesfoundation.org/How-We-Work/General-Information/Open-Access-Policy) private funders consider it; moreover, trial participants want their anonymized data to be made available for further research.2 Let's not forget that systematic reviews and meta-analyses using anonymized data from trial participants have been conducted for years.3 Furthermore, access to these data can facilitate other types of secondary analyses (e.g., subgroup analysis), testing other hypotheses, and conducting the replication of the original analyses and preventing the duplication of trials.
The recommendations of the International Committee of Medical Journal Editors (ICMJE) (http://www.icmje.org/recommendations/) have been pivotal in improving the way scientists communicate the results of medical research, and hundreds of journals now adhere to these guidelines. However, the attitude of the ICMJE (which is not exempt from certain questionable practices regarding the transparency of their activities4) has been less than consistent in this area. In 2016, the ICMJE published a proposal on the transfer of anonymized individual data from clinical trial participants to third parties.5 The ICMJE recognized that sharing these data is an “ethical obligation”, because the “participants have put themselves at risk”, and alluded to growing consensus in this area.5
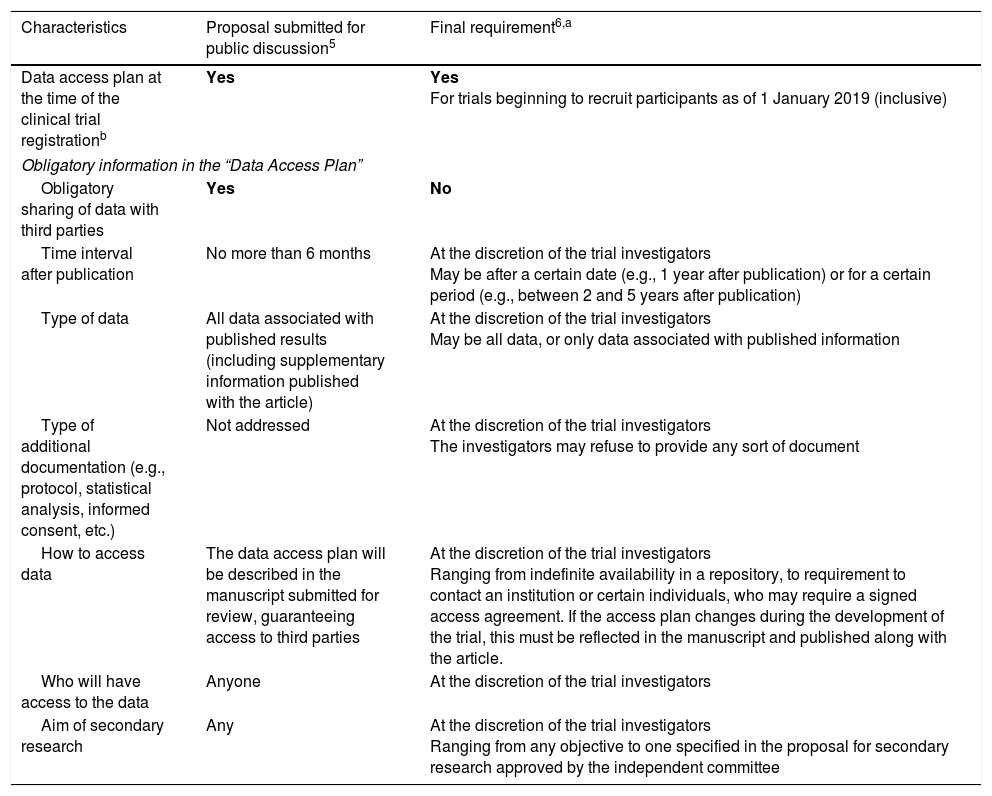
In June 2017, the ICMJE published its final decision, with one significant change of opinion: data sharing has gone from being an obligation to an option.6 Thus, where the proposal required authors to share anonymized patient data with third parties as a condition for publication,5 the final version allowed investigators to share or not to share the data, granting them the freedom to limit access as they saw fit6 (Table 1).
Third-party access to anonymized individual data from clinical trial participants: ICMJE proposal and final requirements.c
| Characteristics | Proposal submitted for public discussion5 | Final requirement6,a |
|---|---|---|
| Data access plan at the time of the clinical trial registrationb | Yes | Yes For trials beginning to recruit participants as of 1 January 2019 (inclusive) |
| Obligatory information in the “Data Access Plan” | ||
| Obligatory sharing of data with third parties | Yes | No |
| Time interval after publication | No more than 6 months | At the discretion of the trial investigators May be after a certain date (e.g., 1 year after publication) or for a certain period (e.g., between 2 and 5 years after publication) |
| Type of data | All data associated with published results (including supplementary information published with the article) | At the discretion of the trial investigators May be all data, or only data associated with published information |
| Type of additional documentation (e.g., protocol, statistical analysis, informed consent, etc.) | Not addressed | At the discretion of the trial investigators The investigators may refuse to provide any sort of document |
| How to access data | The data access plan will be described in the manuscript submitted for review, guaranteeing access to third parties | At the discretion of the trial investigators Ranging from indefinite availability in a repository, to requirement to contact an institution or certain individuals, who may require a signed access agreement. If the access plan changes during the development of the trial, this must be reflected in the manuscript and published along with the article. |
| Who will have access to the data | Anyone | At the discretion of the trial investigators |
| Aim of secondary research | Any | At the discretion of the trial investigators Ranging from any objective to one specified in the proposal for secondary research approved by the independent committee |
The U.S. registry (clinicaltrials.gov) has already been adapted to include a field for reporting on the plan of access to anonymized individual data from clinical trial participants.
The ICMJE is composed of representatives from the following journals: Annals of Internal Medicine; British Medical Journal; Bulletin of the World Health Organization; Deutsches Ärzteblatt (German Medical Journal);); Ethiopian Journal of Health Sciences; JAMA; Journal of Korean Medical Science; New England Journal of Medicine; New Zealand Medical Journal; PLOS Medicine; The Lancet; Revista Médica de Chile; Ugeskrift for Laeger (Danish Medical Journal); U.S. National Library of Science; and the WAME (World Association of Medical Editors).
The ICMJE provides no reasons for their change of opinion, although they do mention their concerns regarding the feasibility of their proposal, the need for multiple resources to enforce the policy, the real or perceived risks to trial participants, the need to protect the interests of clinical trial participants and investigators, and the current lack of mechanisms to mandate universal data sharing.6
No-one claims that requiring all clinical trial sponsors to allow third-party access to their anonymized individual participants’ data is a simple or inexpensive undertaking. Access may be either totally unrestricted, or, conversely, regulated by requiring that the application for access be approved by independent reviewers: for years, this has been the approach of the pharmaceutical industry7 and the UK's Medical Research Council.8 One of the main problems, namely, choosing the mechanisms to be implemented to provide access to numerous clinical trials sponsored by very diverse organizations and institutions, can be resolved with the worldwide Vivli platform. The aim of this project is to link existing platforms and host data from investigators who lack the resources needed to share their data.9
Absolute confidentiality cannot be guaranteed,10 although the ICMJE is right in assuming that data anonymization will protect the rights of study subjects.5 This issue must be addressed by fully informing the participants and by taking all the necessary measures to ensure that individual data are adequately anonymized and correctly managed.1,10
Although not a requirement in the U.S. (http://icmje.org/news-and-editorials/menikoff_icmje_questions_20170307.pdf), participants’ informed consent should be obtained for ongoing and future trials to be used in secondary research.11 It is important to remember that many sponsors currently include a data sharing statement in the patient information sheet, to inform that their anonymized data may be shared with third parties, as part of the process of obtaining informed consent.11 This is especially indicated in research in rare diseases, in which the possibility of identifying participants in the course of a secondary analysis is greater.
Some investigators consider that third-party access to anonymized individual participants’ data might lead to inaccurate analyses, or to analyses designed to discredit the original publication.12 In doing so, they are overlooking the fact that anyone can make a mistake: 35% of re-analyses of clinical trial data led to conclusions (with regard to the type and number of patients to treat) that differed from those of the original authors.13 Furthermore, there is little risk of a secondary analysis distorting the original results, since they are few, and not usually performed to replicate the published results.7,14
Finally, some groups have proposed that clinical trials investigators who share their data be given credit for doing so with the creation of the figure of the “data author”, whose name will appear alongside the author and the co-investigators in any secondary analyses.15
At the present time, anonymized individual participant data sharing for secondary analyses is in some cases mandatory, and in others recommended or facilitated, by funding bodies, including public entities (https://grants.nih.gov/grants/policy/data_sharing/data_sharing_guidance.htm), the pharmaceutical industry and foundations, by investigators (http://www.cochrane.org/about-us/our-governance-and-policies/cochrane-policies/access-data-alltrials, http://nta.nordforsk.org/projects/nta_transparency_report.pdf), and by the Declaration of Helsinki (http://www.wma.net/en/30publications/10policies/b3/index.html), the WHO (http://www.who.int/ictrp/trial_reg/en/), and the UN (https://static1.squarespace.com/static/562094dee4b0d00c1a3ef761/t/57d9c6ebf5e231b2f02cd3d4/1473890031320/UNSG+HLP+Report+FINAL+12+Sept+2016.pdf). The current issues surrounding data sharing are many and wide-ranging, but ultimately have technical solutions. By stepping down from their original proposal, the ICMJE has stopped years of progress towards full transparency in clinical research.
Please cite this article as: Dal-Ré R. Acceso a los datos individuales anonimizados de los participantes en los ensayos clínicos: el cambio radical de opinión de las revistas médicas de mayor prestigio. Arch Bronconeumol. 2018;54:65–67.