Urinothorax, a rare cause of pleural effusion (PE), consists of the presence of urine in the pleural cavity.1–10 It is generally associated with obstructive urinary disease or urinary tract lesion, causing accumulation of urine in the retroperitoneal space, which is filtered to the pleural cavity. Pleural fluid (PF) is usually a transudate, ipsilateral to the lesion.2–9 We report a case of recurrent, difficult-to-manage urinothorax in a patient with stage 4 chronic renal failure, with no apparent obstructive urinary disease. We discuss the pathogenesis of this entity and underline the importance of recognizing this exceptional clinical picture, not previously described in the literature.
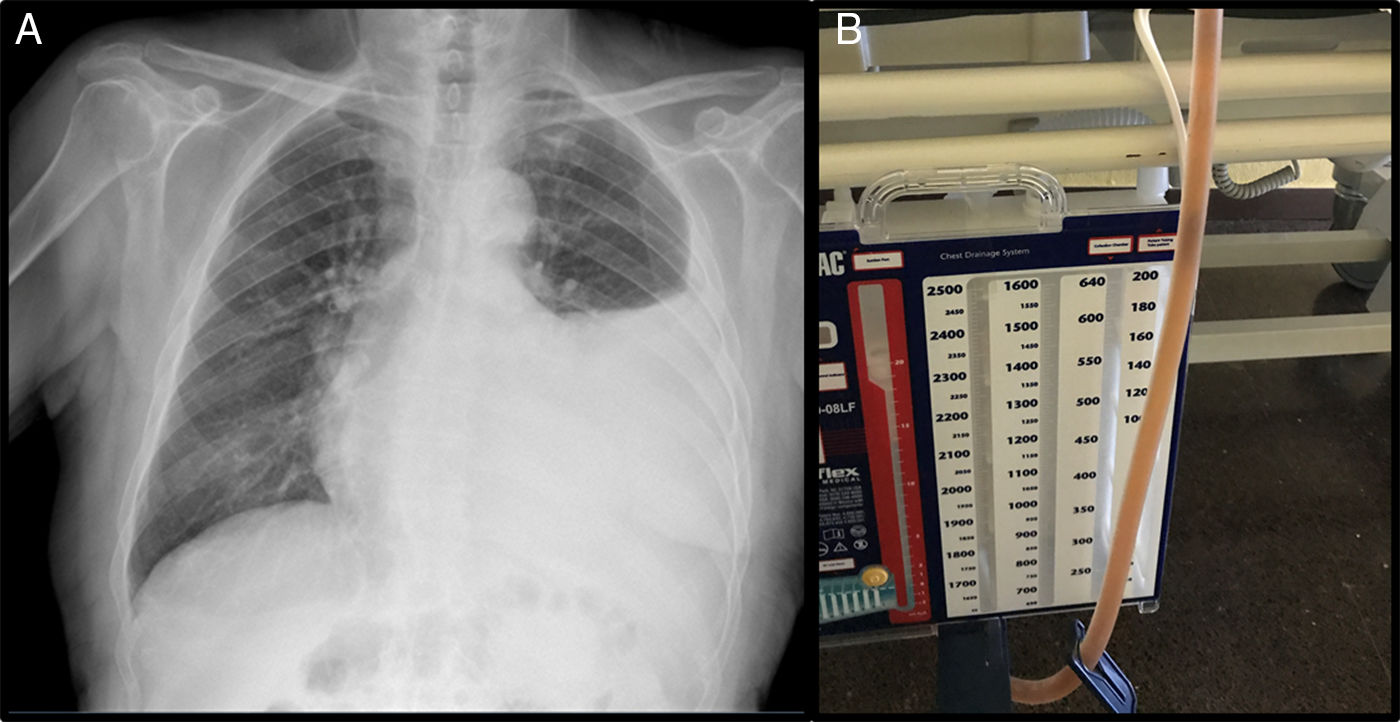
This was an 89-year-old man, non-smoker with a history of arterial hypertension, dyslipidemia, and stage IV chronic kidney disease, with atrophic left kidney due to left renal artery stenosis. He was receiving anticoagulation for chronic atrial fibrillation, and had undergone transurethral resection for benign prostatic hypertrophy, and pulmonary thromboembolism in 2015. He presented in the emergency room for progressive worsening of dyspnea and pain in the left hemithorax, without no previous fever or catarrh. Physical examination showed tachyarrhythmia without murmurs, and auscultation of the lungs revealed signs of left PE. Chest X-ray showed PE occupying the lower two thirds of the hemithorax (Fig. 1). Laboratory tests showed 7000 leukocytes with 82.7% neutrophils; creatinine 2.15mg/dl; total protein 5.56g/dl; albumin 2.70g/dl; LDH: 178IU/l; and CRP: 18mg/l. Ultrasound and computed tomography (CT) of the abdomen and pelvis showed no signs of hydronephrosis nor free liquid, and left kidney measuring 9cm with irregular contour and atrophic appearance, and a small amount of peri-renal fluid. Renal Doppler showed intra-renal flows with increased resistance indices in both renal arteries, indicative of severe stenosis. Chest ultrasound showed moderate PE without septations, and a diagnostic and therapeutic thoracentesis was performed, yielding 1200ml of cloudy, amber-colored PF, smelling of urine. The PF protein/serum protein ratio was 0.69 and the PF LDH/serum LDH ratio was 0.7, confirming the presence of exudate; pH was 7.11. Creatinine in PF was 3.4mg/dl, with PF creatinine/serum creatinine ratio of 1.58, confirming the suspicion of urinothorax. Microbiological cultures and cytology were negative. Thoracentesis and Pleur-evac® were performed 5 times in the following 2 months due to recurrent PE. An intravenous dose of rifampicin was administered, and the PF drained by the Pleur-evac® was confirmed to be copper-colored, so a tunneled pleural catheter was implanted, which remained in place for 2 months, requiring 2 changes of the recipient every week. The patient died of multiple comorbidities and kidney failure not resolved by dialysis.
The first case of urinothorax was described in 1968 by Corriere et al.1 and since then less than 100 reported cases have been reported worldwide. A recent systematic review includes 78 cases.8 Urinothorax is probably underdiagnosed because of its low index of suspicion as a cause of PE.4 Patients with a history of urinothorax usually present with obstructive disease or lesion of the urinary tract,1–9 most frequently benign prostatic hyperplasia, hydronephrosis due to acute obstructive lithiasis, bladder cancer, nephrostomy tube, renal biopsy, etc. The leak in the urinary tract causes extravasation of urine into the pleural space, with or without the previous formation of retroperitoneal urinoma. Urinothorax can be ipsilateral to the renal lesion, although in some occasions it can be bilateral, or even contralateral.8,9 Three mechanisms are involved in its pathogenesis3: the rupture of a urinoma and passage of urine into the pleural space via a fistula; absorption of a urinoma by subdiaphragmatic lymph nodes; or the passage of urine through anatomical defects in the diaphragm. The urinothorax of our patient differs from the cases published due to the absence of a clear history of hydronephrosis caused by obstructive urinary disease or lesion. However, renal artery stenosis is a potential cause of renal atrophy and pyelocalyceal dilation, which can increase intra-renal pressure.11,12 In our case, urinothorax may be explained by an atrophic left kidney and chronic advanced renal failure, and spontaneous extravasation of urine, with leakage through the peri-renal tissue and fascia, passing directly through the diaphragm to the pleural cavity.
Urinothorax often has an amber color and urine-like smell, and from a biochemical point of view displays characteristics of transudate with a pH<7.3 and low protein and glucose ratios,6,7 although in some cases, high levels of LDH lead it to be classified as false exudate.2–8 The main parameter accepted for the confirmation of a diagnosis of urinothorax is a PF creatinine/serum creatinine ratio greater than 1.3,4,7 Other tests that help us to diagnosis this entity are contrast-enhanced CT and intravenous urography, that can detect urinomas and extravasation to the retroperitoneum.2 Renal scintigraphy with radiotracers such as 99mTc DTPA or 99mTc MAG3, reveal the connection between the retroperitoneum and the pleural space.2,10 We can also use dyes such as methylene blue, indigo carmine or, as in our case, rifampicin, that stain the PF immediately after it is injected intravenously. Regarding treatment, removing the urinary tract obstruction will lead to progressive resolution of the PE without the need for drainage,3,4,8 but this approach is indicated only in highly symptomatic patients with massive PE. However, in patients with chronic renal failure, atrophic kidneys, and no clear obstructive disease, therapy can be very complicated, requiring the use of palliative treatments.
Please cite this article as: Molina V, Chiner E, Arlandis M, Bañes S. Urinotórax e insuficiencia renal crónica: una rara asociación. Arch Bronconeumol. 2019;55:105–106.