In the constantly evolving landscape of medical research, multicenter registries of diseases, especially those that are less prevalent or studied, offer several scientific advantages,1 in particular, the possibility of exploring their natural history, the response to specific treatments, and the burden on the health system. In recent decades, inhaled antibiotics have provided hope for patients with chronic airway pathology and chronic bronchial infection2,3 and, as such, have been recommended in various bronchiectasis and COPD guidelines and consensus documents.4–6 However, while clinicians believe that this delivery strategy offers notable advantages over systemic administration in terms of effectiveness and safety, the results of clinical trials have failed to confirm the expected benefits,7,8 and a formal indication in the technical sheet outside the area of cystic fibrosis (CF) has not been granted. As such, many professionals find it difficult or even impossible to prescribe these formulations in certain healthcare settings.
Given the clinical uncertainties surrounding inhaled antibiotics, the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) has sponsored a new initiative to increase evidence on inhaled antibiotics: the creation of REPAIR – the Spanish Registry of Inhaled Antibiotics. This prospective, multicenter registry will include patients who receive inhaled antibiotic therapy as part of their therapeutic regimen for a chronic airway disease, whether bronchiectasis, chronic obstructive pulmonary disease (COPD), or asthma. In the specific case of asthma, this will be the first multicenter series to analyze the benefits and risks of using this type of treatment.9
Registries of treatments that do not have a formal indication and are prescribed on a compassionate use basis, including inhaled antibiotics, differ widely from clinical trials.10 They are designed to provide real-world evidence of effectiveness and safety, allowing researchers to observe long-term trends, variations, and outcomes in a way that controlled trials cannot. They also help identify subgroups of patients who may benefit more from certain treatments, thus contributing to personalized medicine approaches. In addition, they facilitate pharmacoeconomic analyses and guide the allocation of resources. Finally, registries encourage health professionals to collaborate in sharing data and knowledge and promoting research to elucidate the benefits and limitations of these treatments.
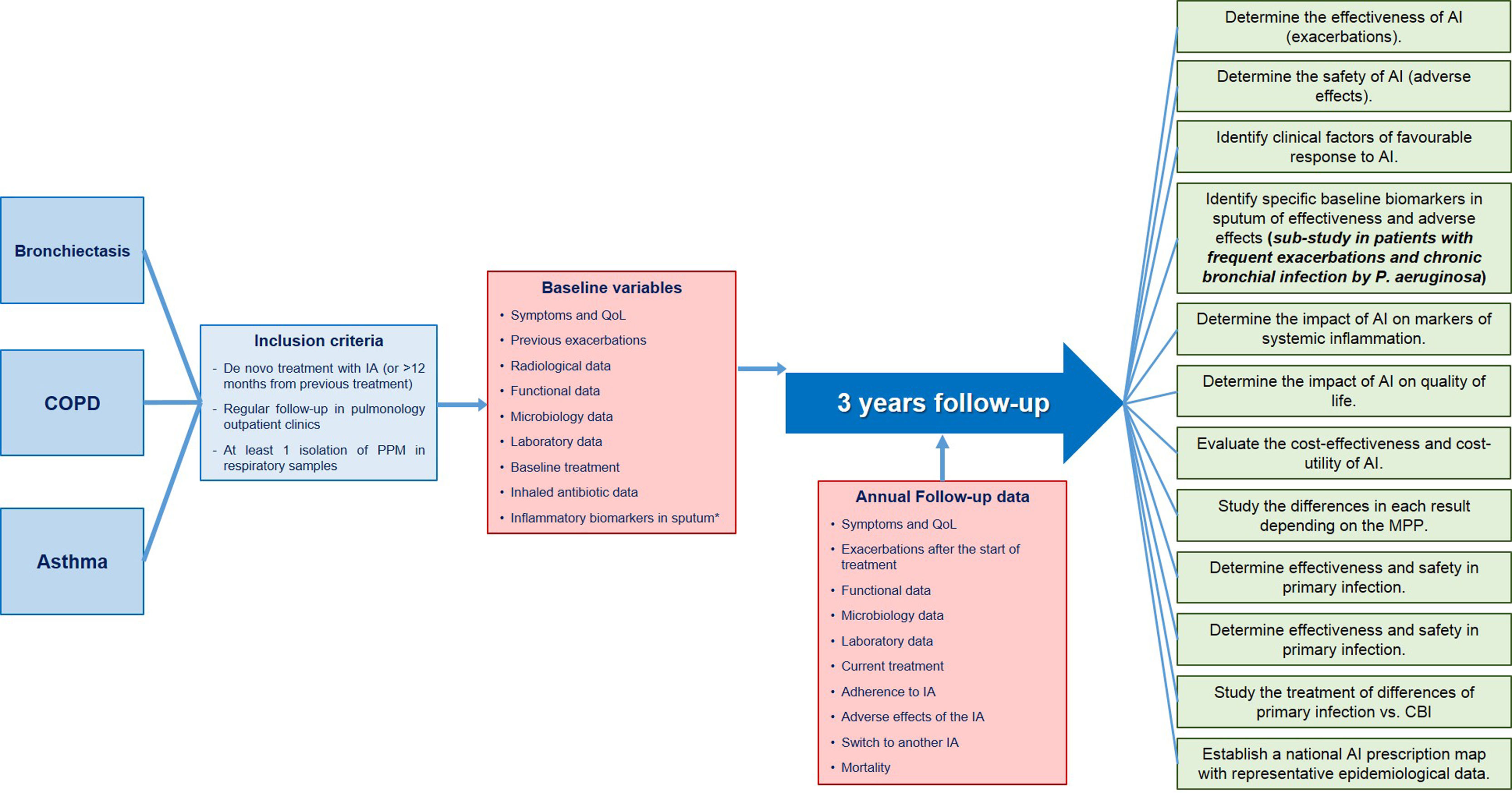
In the specific case of inhaled antibiotics, a multicenter, prospective registry is an immensely promising way of addressing the clinical uncertainties that cloud the horizon of this treatment outside the CF setting. REPAIR is a pioneering worldwide initiative that is being promoted as a project that will fill the gap left by the inconclusive results of clinical trials. By collecting data from multiple centers, the registry can capture the nuances of responses in different patient groups, assess treatment adherence, and evaluate adverse events in a way that transcends the limitations of isolated clinical trials. This holistic perspective will allow for a more complete understanding of the true potential of inhaled antibiotics. The multicenter REPAIR design ensures a representative sample that reflects the heterogeneity of patients receiving inhaled antibiotics. This real-world perspective is invaluable in discerning the actual effectiveness of these treatments beyond the limits of clinical trials and will help identify different subgroups of patients in whom a better or worse response can be anticipated (Fig. 1).
REPAIR summary diagram: patients included, inclusion criteria, baseline data to be collected at the time of inclusion, follow-up data to be collected annually and specific objectives. CBI: chronic bronchial infection; COPD: chronic obstructive pulmonary disease; IA: inhaled antibiotic; PPM: potentially pathogenic microorganisms; QoL: quality of life. * Only in patients included in the sub-study that will be carried out in frequent exacerbating patients with chronic bronchial infection by P. aeruginosa.
One of the main concerns about inhaled antibiotics is their safety profile.11 Clinical trials often fail to examine long-term safety issues and potential interactions with concomitant therapies. REPAIR will notably include an effectiveness and safety sub-study in a group of exacerbating patients with criteria for chronic Pseudomonas aeruginosa infection. In these patients, clinical data and biomarkers in peripheral blood and sputum will be analyzed to identify which factors are associated with a better response or unfavorable therapeutic tolerance. The extensive data collection proposed for REPAIR will allow researchers to delve into the safety aspects in unprecedented detail, providing doctors and patients with a better understanding of the risks associated with inhaled antibiotics.
It is essential to highlight the potential impact of REPAIR in resolving the unanswered questions that plague inhaled antibiotics. By addressing discrepancies between clinical trials and clinical reality, this registry will generate evidence to reshape the narrative surrounding inhaled antibiotics and provide a solid foundation on which doctors can base their decisions and in which patients can place their trust. Spain's pioneering step in establishing the world's first registry of inhaled antibiotics has implications far beyond its borders. The insights gained from REPAIR have the potential to influence treatment protocols and guidelines globally, offering a model for other nations to follow. As we grapple with the complexities of chronic respiratory infections, the collaborative efforts embodied by REPAIR exemplify the spirit of global solidarity in advancing medical knowledge.
However, while REPAIR holds immense promise, it has its challenges. The success of the registry depends on sustained collaboration between participating centers, meticulous data collection, and the commitment of health professionals. Furthermore, the changing landscape of inhaled antibiotics, marked by the introduction in recent years of new formulations and therapies, demands a dynamic and adaptable approach to keep the registry relevant and impactful.
In conclusion, REPAIR is a ray of hope in the field of inhaled antibiotics. Its prospective multicenter design offers a chance to unravel the enigma surrounding the efficacy and safety of inhaled antibiotics and to develop a comprehensive understanding that goes beyond traditional clinical trials. As the first global registry of its kind, Spain's initiative has worldwide significance and paves the way for a new era of evidence-based decision-making in the management of chronic respiratory infections. As we embark on this transformative journey, the medical community is following the progress of REPAIR and eagerly awaiting the answers it offers for the future of inhaled antibiotics.
FundingThere was no funding to carry out this editorial article.
Conflict of interestsThe authors state that they have no conflict of interests.