Exposure to gases and particulate matter released during volcanic eruptions can prove harmful to population health. This paper reports the preliminary results of the ASHES study, aimed at ascertaining the respiratory health effects of the 2021 volcanic eruption in La Palma Island (Spain) on the adult population without previous respiratory disease.
MethodsAmbispective cohort study on the healthy adult population. Three exposure groups were considered: Group 1, high exposure; Group 2, moderate exposure; and Group 3, minor or no exposure. We carried out a descriptive analysis of symptoms during and after the eruption, as well as measure lung function after the eruption (through forced spirometry and diffusing capacity of carbon monoxide).
ResultsThe analysis included 474 subjects: 54 in Group 1, 335 in Group 2, and 85 in Group 3. A significant increase in most symptoms was observed for subjects in the groups exposed during the eruption. After the eruption, this increase remained for some symptoms. There seems to be a dose-response relationship, such that the higher the exposure, the higher the odds ratio. A prebronchodilator FEV1/FVC ratio<70% was observed in 13.0% of subjects in Group 1, 8.6% of subjects in Group 2, and 7.1% of subjects in Group 3.
ConclusionsThis study is the first to report a dose-response relationship between exposure to volcanic eruptions and the presence of symptoms in adults. Furthermore, there is a tendency toward obstructive impairment in individuals with higher exposure.
Volcanic eruptions are natural phenomena that can prove harmful to the health of the exposed population through the release of volcanic gases, aerosols, ash, and smog, among other substances, into the atmosphere.1–3 The enormous heterogeneity in the presentation of these eruptions, coupled with their relatively low frequency and duration in time render the study of their repercussions on human beings complicated.
To date, numerous studies have analyzed the impact of volcanic eruptions on human health. These studies have reported an increase in the incidence of different diseases, ranging from mental to cardiovascular, as a consequence of exposure to pollutants released during the course of such eruptions.2 The respiratory system has been identified as one of the most affected by the worsening air quality caused by volcanic eruptions, and a number of studies have analyzed the respiratory effects, both short- and long-term. Different systematic reviews4–7 have identified the presence of pulmonary inflammation and respiratory symptoms, such as cough or dyspnea, development of bronchitis and asthma, as well as exacerbation of preexisting respiratory diseases, associated with exposures caused by volcanic eruptions. Most of the studies conducted have a short follow-up period, generally of weeks, or no follow-up at all,8 so that the evidence available on long-term effects is limited.5 Silicosis and chronic obstructive pulmonary disease (COPD) have been identified as the long-term consequences of potentially most concern.5 Studies undertaken in Iceland and Japan are those that have logged the longest follow-up times.9–11 Although Icelandic studies have observed an increased risk of exposure-related symptoms 3–4 years after the eruption,9,10 no incident cases of eruption-related pulmonary diseases have been reported to date. A Japanese study with a 6-year follow-up has reported a higher frequency of symptoms among subjects with long-term exposure but no deterioration in lung function.11
On September 19, 2021, the Tajogaite volcano (Cumbre Vieja ridge, La Palma Island, Spain) began erupting, and the eruption lasted for 85 days. During this period, the emission of volcanic lava, ashes, and gases of differing nature and composition required the urgent evacuation of the population living in the surrounding areas of the volcano and along the envisaged path of the resulting lava flows. It should be noted that this eruption occurred very close to densely populated areas, and that the air quality on the island of La Palma is normally characterized by very low levels of anthropogenic pollution. The volcano ejected large quantities of fine particles of ash and sulfur dioxide, which in the final stages of the eruption amounted to as much as 50,000 tons per day. This gave rise to episodes of extremely adverse air quality which exceeded – at times by as much as fourfold – the daily PM10 limit value of 50μg/m3 set by the prevailing legislation,12–14 and made it necessary to place part of the population under mandatory confinement.15
In October 2021, the Spanish Society of Pneumology and Thoracic Surgery (Sociedad Española de Neumología y Cirugía Torácica/SEPAR) decided to sponsor the Analysis of Exposure and Respiratory Health Effects of Volcanic Eruption in the Canary Islands (“ASHES study”),16 for the purpose of analyzing the health impact of air pollution caused by the Tajogaite volcanic eruption, in the adult population, pediatric population, respiratory patients, and highly exposed subjects. The aim of this paper is thus to report the preliminary results of the ASHES study on the symptoms and lung function of the healthy adult population.
MethodsStudy design and settingThe ASHES project is a multidesign study, the study protocol has been described in detail in a previous paper.16
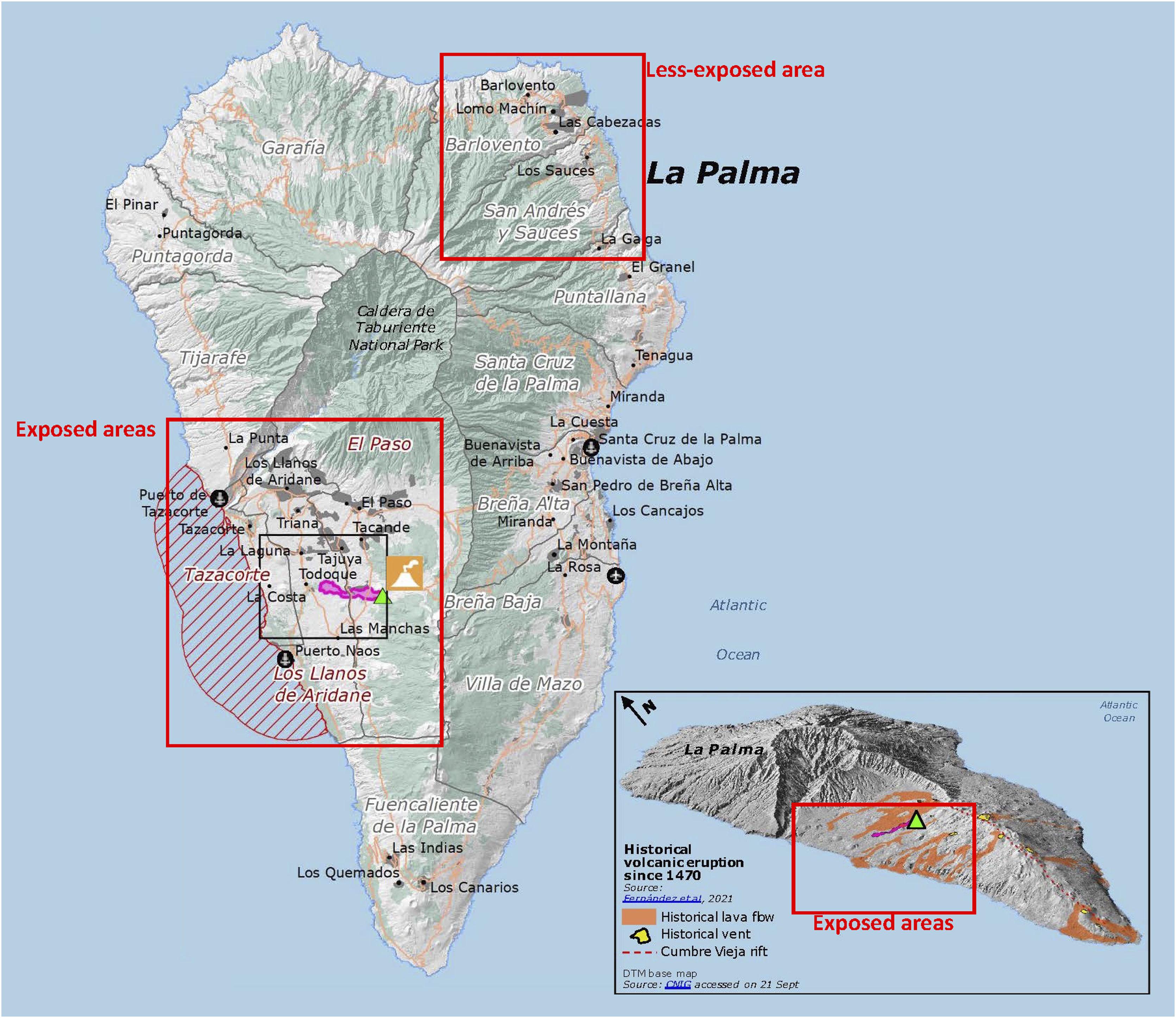
This study was conducted on La Palma Island (Spain) (Fig. 1). The island is divided along its length by a mountain chain, with the eruption occurring in the southwestern segment, thus making it possible to define populations with different degrees of exposure.
Map showing La Palma with the high-exposure and low-exposure areas.18
This study contains three groups of subjects, defined by reference to their respective levels of exposure: high exposure or Group 1 (enjoying access to the exclusion zone during the eruption); moderate exposure or Group 2 (residents of municipalities near the volcano, namely Los Llanos de Aridane, Tazacorte and El Paso); and minor/no exposure or Group 3 (residents of municipalities situated farthest from the volcano, namely Barlovento and San Andrés y Los Sauces). Group 3 subjects were residents of the northwestern area of the island, where the highest mountain divide is situated. We compared the levels of different environmental pollutants in each area during the eruption, in order to select the towns of residence of each exposure group.
The recruitment period for all study participants included in this paper lasted from March 2022 to July 2023.
Adult participants were recruited by convenience sampling, with the study being publicly announced at strategic points in the selected municipalities. For inclusion purposes, we required all participants to be aged 18 years or over and have no history of previous respiratory diseases. Group 1 subjects were required to have had access to the exclusion zone for at least one month. Group 2 and 3 subjects were required to have been registered residents of the municipalities chosen for recruitment, and not to have changed their place of residence during the eruption, or if they had done so (due to forced evacuation), to have moved to a place lying within the same exposure zone.
The study protocol was approved on 24 February 2022 under code no. CHUC_2022_14 (ASHES-1) by the Drug Research Ethics Committee of the Canarias University Teaching Hospital Complex (Province of Santa Cruz de Tenerife). All subjects who agreed to participate signed an informed consent form.
Data-collectionThe following three different data sources were used in the study: a personal questionnaire-based interview conducted by a trained interviewer; lung function tests; blood and urine samples and nasal swabs taken from all subjects. This paper reports the analysis of the data collected from questionnaires and the results of lung function tests.
The main questionnaire was purpose-designed for this study and included sociodemographic data, dwelling characteristics, smoking and exposure to second-hand smoke, among others. This questionnaire is based on the 2019 guidelines of the International Volcanic Health Hazard Network (IVHHN) for conducting epidemiological studies related to volcanic eruptions.17 In addition, validated questionnaires were used to ascertain respiratory symptoms during and after the eruption (European Community Respiratory Health Survey/ECRHS III) and degree of dyspnea (modified Medical Research Council/mMRC scale).
Participants underwent forced prebronchodilator spirometry and lung diffusing capacity (diffusing capacity of carbon monoxide/DLCO) tests. The lung function tests were performed in accordance with European Respiratory Society19 and SEPAR guidelines.
In any case where spirometry yielded abnormal values, postbronchodilator spirometry was performed. In these cases, we determined whether there had been a positive bronchodilator test, defined as a difference of more than 12% between pre- and postbronchodilator values.
Statistical analysisWe performed a descriptive analysis of the data, comparing the main characteristics of the participants according to their exposure group, using the Chi-squared test for qualitative variables and ANOVA for quantitative variables. For analysis of the symptoms, we performed a number of logistic regressions, adjusted for sex, age, smoking and Charlson comorbidity index, in which the independent variable was having reported the symptom (yes/no), and the dependent variable was the exposure group. The reference category for the dependent variable was Group 3, the group with minor/no exposure. Odds ratios (ORs) along with their 95% confidence intervals are presented. The number of eruption-related symptoms reported by the subjects were calculated and compared by exposure group, using the chi-squared test for linear trend. Lastly, we also calculated and compared the differences between the number of symptoms reported during and after the eruption.
For analysis of lung function, a descriptive analysis was performed by exposure group, using the Chi-squared test for linear trend to analyze the differences between groups; and the same analysis was performed by sex. We fitted logistic regressions in which the independent variable was a measure of lung function (FEV1, FVC, FEV1/FVC and DLCO, all of them prebronchodilator) and the dependent variable was the exposure group. These regressions were adjusted by sex, age, smoking and Charlson comorbidity index. Statistical significance was set at p<0.05. All analyses were performed using the Stata v17.0 computer software package.
ResultsTo date, a total of 476 adults have been recruited, with 2 being excluded for not providing information about the exposure group to which they belonged. The sample analyzed ultimately comprised 474 healthy adults: 54 belonging to Group 1 (high exposure), 335 belonging to Group 2 (moderate exposure), and 85 belonging to Group 3 (control group, no/minor exposure).
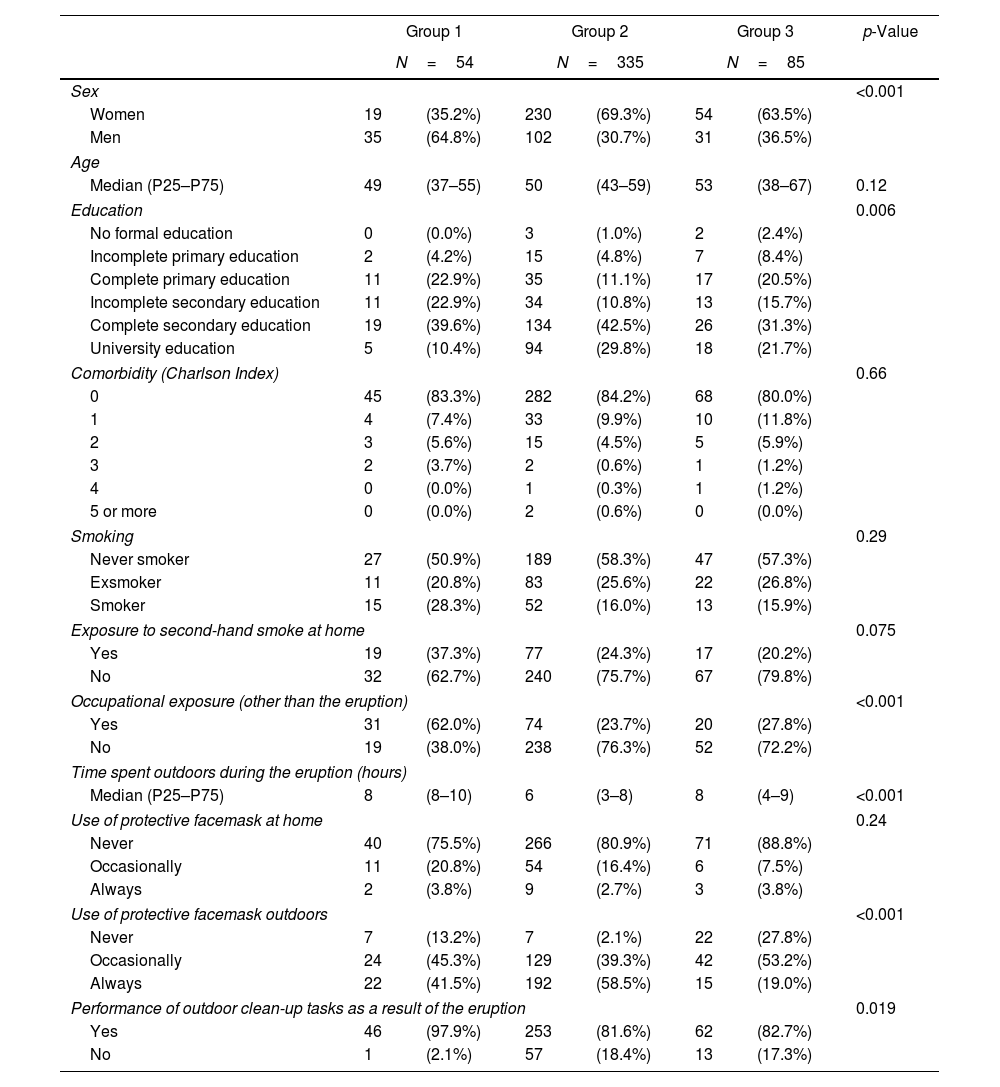
Table 1 shows the main characteristics of the sample by exposure group. Differences between groups were observed in several variables. Group 1 had fewer women than did Groups 2 and 3. In general, the subjects in Groups 2 and 3 had a higher educational level than those belonging to Group 1. Likewise, a higher proportion of Group 1 subjects had undergone occupational exposure to toxic substances, and almost all (97.9%) reported having performed outdoor tasks involving the clearing and cleaning up of volcanic ash.
Main characteristics of study participants.
| Group 1 | Group 2 | Group 3 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| N=54 | N=335 | N=85 | |||||
| Sex | <0.001 | ||||||
| Women | 19 | (35.2%) | 230 | (69.3%) | 54 | (63.5%) | |
| Men | 35 | (64.8%) | 102 | (30.7%) | 31 | (36.5%) | |
| Age | |||||||
| Median (P25–P75) | 49 | (37–55) | 50 | (43–59) | 53 | (38–67) | 0.12 |
| Education | 0.006 | ||||||
| No formal education | 0 | (0.0%) | 3 | (1.0%) | 2 | (2.4%) | |
| Incomplete primary education | 2 | (4.2%) | 15 | (4.8%) | 7 | (8.4%) | |
| Complete primary education | 11 | (22.9%) | 35 | (11.1%) | 17 | (20.5%) | |
| Incomplete secondary education | 11 | (22.9%) | 34 | (10.8%) | 13 | (15.7%) | |
| Complete secondary education | 19 | (39.6%) | 134 | (42.5%) | 26 | (31.3%) | |
| University education | 5 | (10.4%) | 94 | (29.8%) | 18 | (21.7%) | |
| Comorbidity (Charlson Index) | 0.66 | ||||||
| 0 | 45 | (83.3%) | 282 | (84.2%) | 68 | (80.0%) | |
| 1 | 4 | (7.4%) | 33 | (9.9%) | 10 | (11.8%) | |
| 2 | 3 | (5.6%) | 15 | (4.5%) | 5 | (5.9%) | |
| 3 | 2 | (3.7%) | 2 | (0.6%) | 1 | (1.2%) | |
| 4 | 0 | (0.0%) | 1 | (0.3%) | 1 | (1.2%) | |
| 5 or more | 0 | (0.0%) | 2 | (0.6%) | 0 | (0.0%) | |
| Smoking | 0.29 | ||||||
| Never smoker | 27 | (50.9%) | 189 | (58.3%) | 47 | (57.3%) | |
| Exsmoker | 11 | (20.8%) | 83 | (25.6%) | 22 | (26.8%) | |
| Smoker | 15 | (28.3%) | 52 | (16.0%) | 13 | (15.9%) | |
| Exposure to second-hand smoke at home | 0.075 | ||||||
| Yes | 19 | (37.3%) | 77 | (24.3%) | 17 | (20.2%) | |
| No | 32 | (62.7%) | 240 | (75.7%) | 67 | (79.8%) | |
| Occupational exposure (other than the eruption) | <0.001 | ||||||
| Yes | 31 | (62.0%) | 74 | (23.7%) | 20 | (27.8%) | |
| No | 19 | (38.0%) | 238 | (76.3%) | 52 | (72.2%) | |
| Time spent outdoors during the eruption (hours) | |||||||
| Median (P25–P75) | 8 | (8–10) | 6 | (3–8) | 8 | (4–9) | <0.001 |
| Use of protective facemask at home | 0.24 | ||||||
| Never | 40 | (75.5%) | 266 | (80.9%) | 71 | (88.8%) | |
| Occasionally | 11 | (20.8%) | 54 | (16.4%) | 6 | (7.5%) | |
| Always | 2 | (3.8%) | 9 | (2.7%) | 3 | (3.8%) | |
| Use of protective facemask outdoors | <0.001 | ||||||
| Never | 7 | (13.2%) | 7 | (2.1%) | 22 | (27.8%) | |
| Occasionally | 24 | (45.3%) | 129 | (39.3%) | 42 | (53.2%) | |
| Always | 22 | (41.5%) | 192 | (58.5%) | 15 | (19.0%) | |
| Performance of outdoor clean-up tasks as a result of the eruption | 0.019 | ||||||
| Yes | 46 | (97.9%) | 253 | (81.6%) | 62 | (82.7%) | |
| No | 1 | (2.1%) | 57 | (18.4%) | 13 | (17.3%) | |
P: percentile.
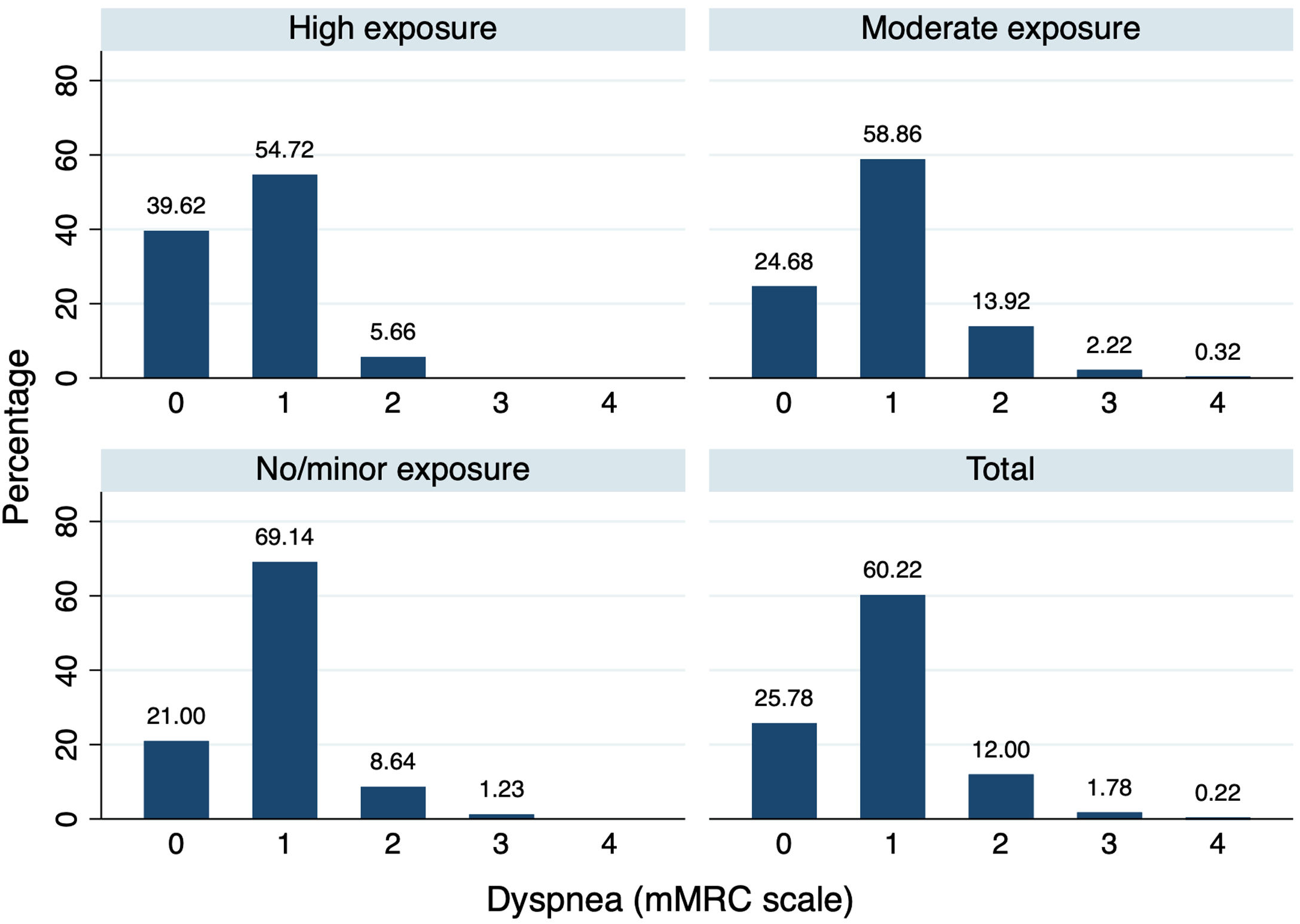
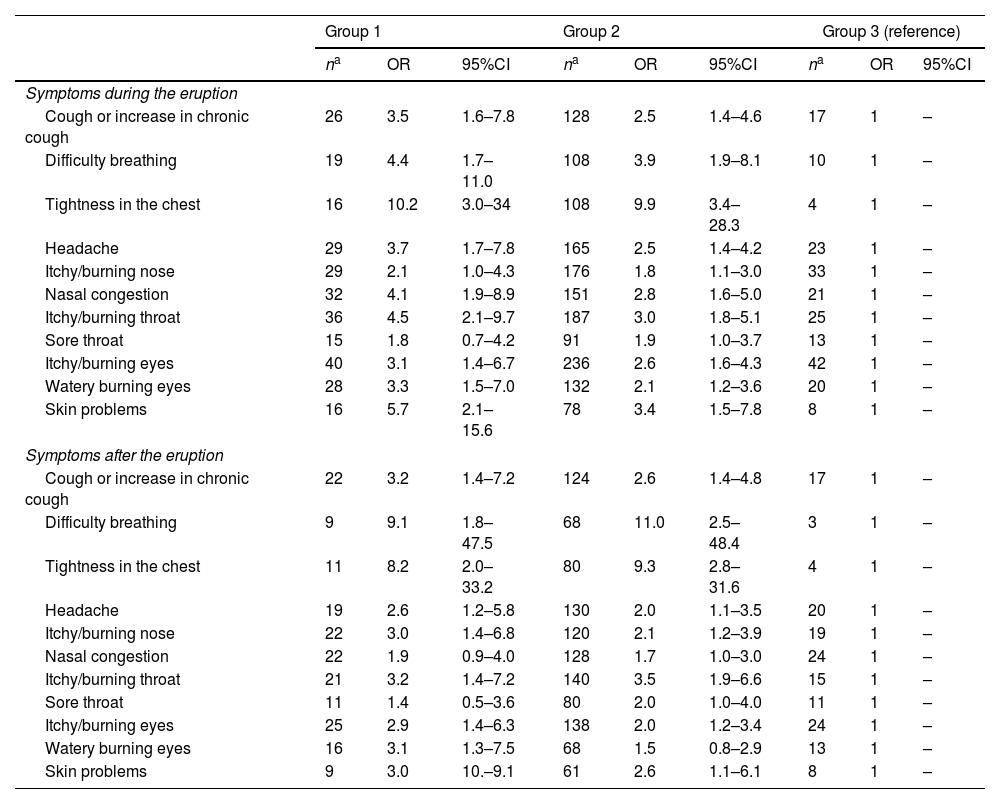
Table 2 lists the results of the adjusted logistic regressions showing symptoms during and after the eruption, by exposure group, with Group 3 being the comparison group. During the eruption, a significant increased risk was observed for all symptoms except for itchy/burning nose and sore throat. When it came to symptoms recorded after the eruption, a significant increased risk was also observed in exposed subjects for all symptoms, except for nasal congestion, sore throat, and skin problems. A dose-response relationship appeared to be evident in the presence of symptoms, such that the higher the level of exposure, the higher the OR, mainly with respect to symptoms reported during the eruption. This relationship seemed to be maintained in the case of some symptoms reported after the end of the eruption, such as cough or itchy/burning nose. When the degree of dyspnea was analyzed, no differences were observed between groups (p=0.17) (Fig. 2).
Logistic regression of symptoms during and after the eruption, by exposure group.
| Group 1 | Group 2 | Group 3 (reference) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| na | OR | 95%CI | na | OR | 95%CI | na | OR | 95%CI | |
| Symptoms during the eruption | |||||||||
| Cough or increase in chronic cough | 26 | 3.5 | 1.6–7.8 | 128 | 2.5 | 1.4–4.6 | 17 | 1 | – |
| Difficulty breathing | 19 | 4.4 | 1.7–11.0 | 108 | 3.9 | 1.9–8.1 | 10 | 1 | – |
| Tightness in the chest | 16 | 10.2 | 3.0–34 | 108 | 9.9 | 3.4–28.3 | 4 | 1 | – |
| Headache | 29 | 3.7 | 1.7–7.8 | 165 | 2.5 | 1.4–4.2 | 23 | 1 | – |
| Itchy/burning nose | 29 | 2.1 | 1.0–4.3 | 176 | 1.8 | 1.1–3.0 | 33 | 1 | – |
| Nasal congestion | 32 | 4.1 | 1.9–8.9 | 151 | 2.8 | 1.6–5.0 | 21 | 1 | – |
| Itchy/burning throat | 36 | 4.5 | 2.1–9.7 | 187 | 3.0 | 1.8–5.1 | 25 | 1 | – |
| Sore throat | 15 | 1.8 | 0.7–4.2 | 91 | 1.9 | 1.0–3.7 | 13 | 1 | – |
| Itchy/burning eyes | 40 | 3.1 | 1.4–6.7 | 236 | 2.6 | 1.6–4.3 | 42 | 1 | – |
| Watery burning eyes | 28 | 3.3 | 1.5–7.0 | 132 | 2.1 | 1.2–3.6 | 20 | 1 | – |
| Skin problems | 16 | 5.7 | 2.1–15.6 | 78 | 3.4 | 1.5–7.8 | 8 | 1 | – |
| Symptoms after the eruption | |||||||||
| Cough or increase in chronic cough | 22 | 3.2 | 1.4–7.2 | 124 | 2.6 | 1.4–4.8 | 17 | 1 | – |
| Difficulty breathing | 9 | 9.1 | 1.8–47.5 | 68 | 11.0 | 2.5–48.4 | 3 | 1 | – |
| Tightness in the chest | 11 | 8.2 | 2.0–33.2 | 80 | 9.3 | 2.8–31.6 | 4 | 1 | – |
| Headache | 19 | 2.6 | 1.2–5.8 | 130 | 2.0 | 1.1–3.5 | 20 | 1 | – |
| Itchy/burning nose | 22 | 3.0 | 1.4–6.8 | 120 | 2.1 | 1.2–3.9 | 19 | 1 | – |
| Nasal congestion | 22 | 1.9 | 0.9–4.0 | 128 | 1.7 | 1.0–3.0 | 24 | 1 | – |
| Itchy/burning throat | 21 | 3.2 | 1.4–7.2 | 140 | 3.5 | 1.9–6.6 | 15 | 1 | – |
| Sore throat | 11 | 1.4 | 0.5–3.6 | 80 | 2.0 | 1.0–4.0 | 11 | 1 | – |
| Itchy/burning eyes | 25 | 2.9 | 1.4–6.3 | 138 | 2.0 | 1.2–3.4 | 24 | 1 | – |
| Watery burning eyes | 16 | 3.1 | 1.3–7.5 | 68 | 1.5 | 0.8–2.9 | 13 | 1 | – |
| Skin problems | 9 | 3.0 | 10.–9.1 | 61 | 2.6 | 1.1–6.1 | 8 | 1 | – |
Adjusted by sex, age and Charlson comorbidity index.
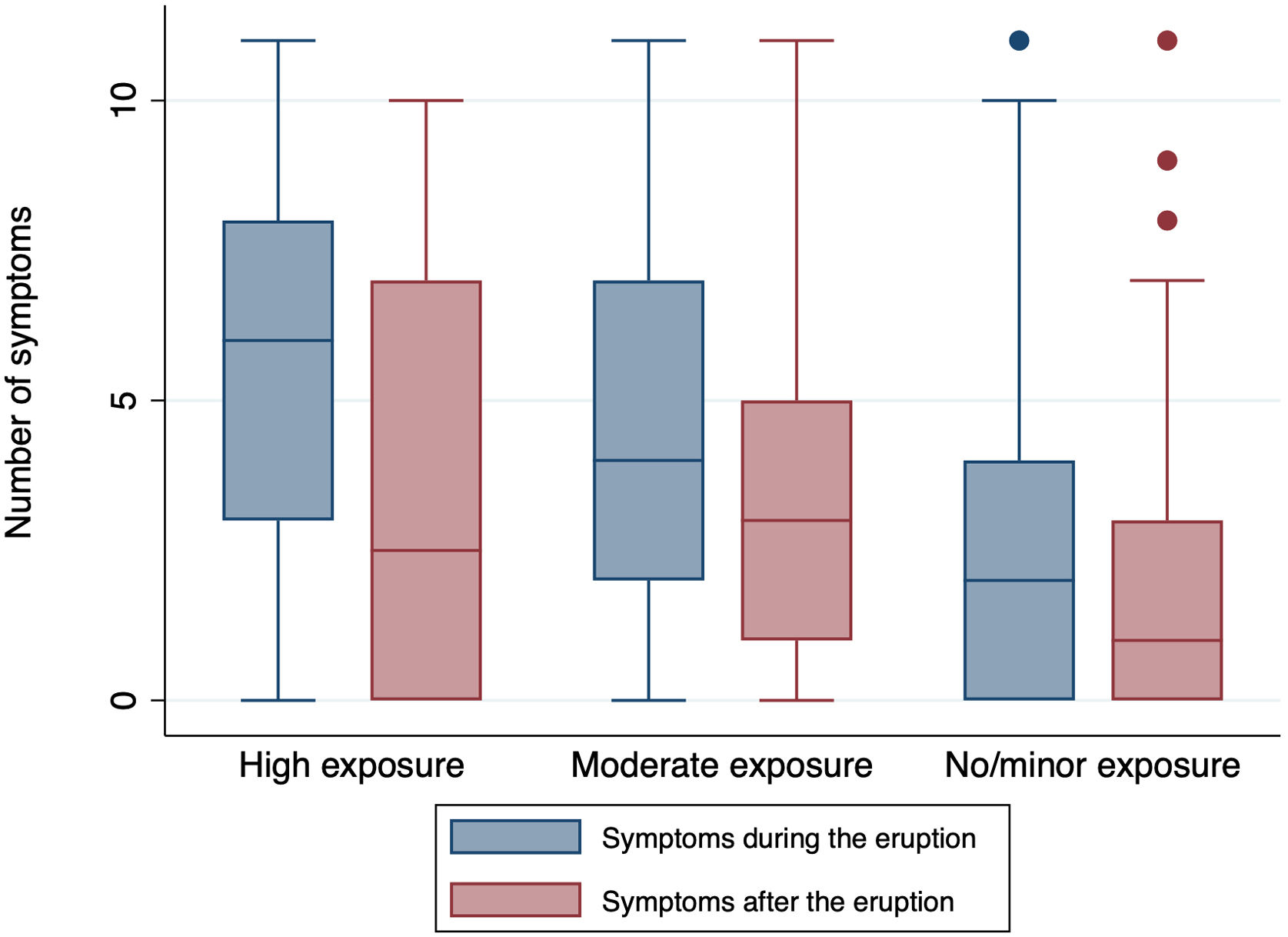
The symptoms reported during and after the eruption were analyzed according to sex. There were no differences by sex, only in sore throat. In contrast, in group 2, most symptoms were reported more frequently by women compared to men (Supplementary Table 1). The number of symptoms associated the volcanic eruption was analyzed by exposure group (Fig. 3). Group 1 subjects reported a median of 6 symptoms (P25–P75 3–8) during the eruption, whereas Group 2 subjects reported a median of 42–7 and Group 3 a median of 2 (0–4) symptoms. The differences between exposure groups were statistically significant for the number of symptoms both before and after the eruption (p<0.001). By sex, women presented with a higher number of symptoms than did men in all exposure groups, though statistical significance was only observed in Group 2, in which women had a median of 5 symptoms and men a median of 3.5 symptoms (p=0.01) (Supplementary Fig. 1). After the eruption, the median number of symptoms fell statistically significantly in all exposure groups, though the greatest fall was observed in Group 1 (p=0.03).
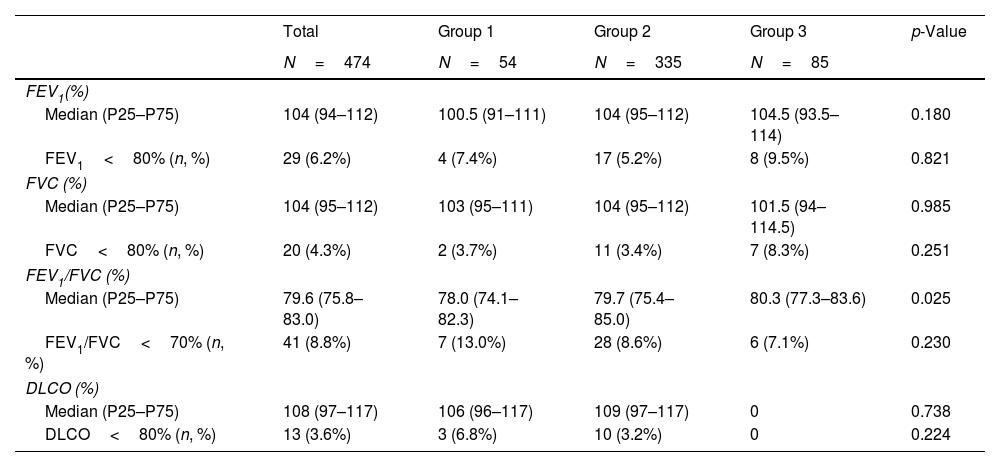
A total of 465 participants underwent prebronchodilator spirometry (missing data: 11). A prebronchodilator FEV1/FVC value<70% (obstructive impairment in spirometry) was registered by 7 (13.0%) subjects in Group 1, 28 (8.6%) in Group 2, and 6 (7.1%) in Group 3. While the differences between groups were not significant (p=0.224), the presence of obstructive impairment observed for the high exposure group (Group 1) was almost double that for the group with minor or no exposure (Group 3). Furthermore, the median FEV1/FVC percentage described a statistically significant rising trend, with the lowest value (p=0.03) being recorded in Group 1. According to sex, Supplementary Fig. 3 shows that the median FEV1/FVC percentage is lower in women than in men in exposure Groups 1 and 2. This difference is statistically significant in the case of highly exposed subjects (p=0.04), although in the moderately exposed group it is almost significant (p=0.06).
Of subjects who underwent spirometry, 6.2% (n=29) presented with abnormal values in pre-bronchodilator FEV1 and 4.3% (n=20) in prebronchodilator FVC. Table 3 shows this percentage by exposure group. The differences between groups were not significant (p>0.05, in all cases). Supplementary Table 2 shows the adjusted logistic regressions, none of the calculated ORs was significant. The postbronchodilator values for a limited number of subjects (n=100) can be seen in Supplementary Table 3, both overall and by exposure group. Spirometric patterns with pre- and postbronchodilator values can be consulted in Supplementary Table 4. A total of 20 subjects (19.8% of whom underwent postbronchodilator spirometry) had differences of more than 12% between pre- and postbronchodilator spirometry values (positive bronchodilator test).
Summary of prebronchodilator spirometry parameters and percentage of subjects with abnormal values.
| Total | Group 1 | Group 2 | Group 3 | p-Value | |
|---|---|---|---|---|---|
| N=474 | N=54 | N=335 | N=85 | ||
| FEV1(%) | |||||
| Median (P25–P75) | 104 (94–112) | 100.5 (91–111) | 104 (95–112) | 104.5 (93.5–114) | 0.180 |
| FEV1<80% (n, %) | 29 (6.2%) | 4 (7.4%) | 17 (5.2%) | 8 (9.5%) | 0.821 |
| FVC (%) | |||||
| Median (P25–P75) | 104 (95–112) | 103 (95–111) | 104 (95–112) | 101.5 (94–114.5) | 0.985 |
| FVC<80% (n, %) | 20 (4.3%) | 2 (3.7%) | 11 (3.4%) | 7 (8.3%) | 0.251 |
| FEV1/FVC (%) | |||||
| Median (P25–P75) | 79.6 (75.8–83.0) | 78.0 (74.1–82.3) | 79.7 (75.4–85.0) | 80.3 (77.3–83.6) | 0.025 |
| FEV1/FVC<70% (n, %) | 41 (8.8%) | 7 (13.0%) | 28 (8.6%) | 6 (7.1%) | 0.230 |
| DLCO (%) | |||||
| Median (P25–P75) | 108 (97–117) | 106 (96–117) | 109 (97–117) | 0 | 0.738 |
| DLCO<80% (n, %) | 13 (3.6%) | 3 (6.8%) | 10 (3.2%) | 0 | 0.224 |
P: percentile.
The results of this study highlight, first, that emissions produced by volcanic eruptions lead to a significant increase in respiratory symptomatology which, moreover, is seemingly dose-dependent. Subjects with the highest exposure show a higher likelihood of presenting with symptoms. Second, there is a trend toward a greater impairment in the most exposed as compared with the least exposed subjects. Indeed, some of the most exposed subjects were observed to display an obstructive pattern after the volcanic eruption.
This study indicates a higher frequency of self-reported eruption-related symptoms in subjects exposed to the poor air quality caused by these events, compared with subjects with no or minor exposure. A previous study on the effects of the eruption of the Tajogaite volcano on subjects living near and far from the volcano found that those living nearby were twice as likely to develop respiratory symptoms.20 Other previous studies, conducted in the context of different volcanic eruptions, have also observed an increase in symptoms related with exposure among adult subjects.9,21–25 The results observed by our study are similar to those previously reported in recent eruptions, such as that of the Icelandic volcano, Eyjafjallajökull, in 2010. In that study an increase was observed in short-term symptomatology, such as cough or tightness in the chest.21 Furthermore, the symptomatology was measured using the same scale as in our study, and the symptom evaluation date was also similar (6–15 months after the eruption had ended), something that allows for comparability of results. Another study which analyzed the same volcanic eruption, with a 3-year post-eruption follow-up, reported that subjects who were residing in areas near the eruption displayed a higher risk of respiratory symptoms, suggesting that in the most exposed subjects, the effects of exposure continue to be felt in the long-term.9 Additionally, our study observed a dose-response gradient in symptomatology, such that the higher the subjects’ degree of exposure, the higher the risk of they report symptoms. This dose-response gradient has not been previously observed in studies targeting adults.11 That said, however, a study conducted in Japan on children divided into four exposure groups, reported that the prevalence of certain symptoms, such as cough, rose, as the degree of the children's exposure increased.26
Worse lung function appeared to be present among the subjects most exposed to the eruption. Hence, highly and moderately exposed subjects registered lower FEV1/FVC values than did the least exposed subjects. Furthermore, 3.6% of the sample displayed changes in carbon monoxide diffusing capacity, and in all cases belonged to the exposed group. This result could indicate pulmonary alveolar and/or vascular involvement.
Previous evidence relating to the association between a worsening of lung function and exposure to volcanic eruptions is not conclusive. Studies undertaken in Iceland27 and Japan11,24 did not report a worsening of lung function in adult subjects. However, a study undertaken in Mexico on a limited number of nonsmokers (n=35), observed an acute worsening of lung function which reverted to normal values with time, except for the FEV1/FVC ratio which continued to fall after 7 months of exposure.25 The involvement of farmers in the Mexican study suggests that potential confounding factors, such as occupational exposure to organic dust from stables, pesticides or bioaerosols emitted by hay, cannot be disregarded. These factors have been linked to heightened respiratory symptoms and, in certain investigations, to impaired lung function.28 A subgroup of participants (n=202) in the Icelandic study showed worsened lung function among those exposed, despite similar GOLD grades in control and exposed groups.29 Differences from previous studies may be due to dispersion of exposed subjects, their proximity to the eruptive cone, or ash composition. Our study underscores a stronger biological plausibility for associating lung function decline with proximity to the eruptive cone, given the proximity of the exposed population to it.
It is observed that women have lower lung function than men in the results obtained by sex. Previous studies have concluded that women have smaller lungs than men, resulting in a smaller number of bronchi and alveolar surface area, as well as lower airflow values.30 It is possible that, as a result, exposed women may have a greater decrease in FEV1/FVC. However, this cannot be confirmed because we do not have spirometric data from subjects before the eruption. In addition, in our study, women reported a greater number of symptoms than men in the moderate exposure group. Ishigami et al.31 reported similar results: women exposed to SO2 from a volcanic eruption in Japan were at higher risk of presenting symptoms than men. The authors raised the possibility that women may be more sensitive to irritant gases than men. This phenomenon has also been seen with other risk factors, such as tobacco use. Authors, such as Langhammer et al.,32 reported that women were more susceptible to the effects of smoking than men, with a higher frequency of symptoms. This greater susceptibility may be due to cyclic hormonal fluctuations and the possible antiestrogenic effect of tobacco. These factors may also increase women's susceptibility to pollution from volcanic eruptions.
This study has several advantages associated with the characteristics of the volcanic eruption site. Firstly, the island of La Palma experiences low levels of anthropogenic pollution due to its location, resulting in minimal pollution from heating or road traffic, and lacks significant industrial activity. This facilitates attributing causality to the volcanic eruption compared to eruptions in more polluted areas. Additionally, the eruption occurred close to the densely populated part of the island, facilitating exposure of the population to volcanic ash and gases, with 7000 people evacuated. The study design, based on IVHHN guidelines,17 includes three groups with different levels of exposure, enabling dose–response associations. Detailed data were collected, including spirometry tests and questionnaires, allowing for comprehensive analysis.
This study also has limitations. Firstly, recruitment began after the eruption ended, potentially diluting the effect in exposed subjects or introducing memory bias regarding symptoms during the eruption. Data collection prioritized highly or moderately exposed subjects over those with little exposure, assuming their lower exposure remained stable over time. Additionally, convenience sampling may have introduced selection bias. Exposure data from monitoring stations in the volcanic area were not included, as highly exposed subjects would leave no trace and assigning exposure based on dwellings could lead to collinearity issues. The data in this study were self-reported, and no clinical examinations were conducted. Therefore, there may be discrepancies between the self-reported symptoms and the symptoms registered through clinical examination. Lastly, there were fewer subjects in the lowest exposure group due to later recruitment, although the impact on results is deemed minimal.
In conclusion, the results of this study are in line with previously available evidence on the increase in symptomatology associated with volcanic eruptions. Furthermore, this study observed a dose–response relationship not previously reported in adults. With respect to deterioration in lung function, the preliminary results of this study suggest a relationship between this and exposure, though this is something that will have to be confirmed when the entire recruited sample becomes available. These results underscore the importance of health prevention and surveillance in populations exposed to volcanic eruptions, and particularly in workers from highly exposed areas. Early identification and treatment of exposure-related symptoms could help mitigate the adverse health effects associated with these disasters. Even so, more research is needed for a better understanding of the long-term effects of exposure to volcanic eruptions, something that is envisaged in the ASHES study with the continuation of the follow-up of participants and analysis of the impact on other subgroups (children or subjects with previous respiratory diseases).
Ethical considerationsThis study was implemented following good practice guidelines and the Declaration of Helsinki. The study protocol was approved on 24 February 2022 under code no. CHUC_2022_14 (ASHES-1) by the Drug Research Ethics Committee of the Canarias University Teaching Hospital Complex (Province of Santa Cruz de Tenerife).
Data availabilityThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
FundingThis study is funded by the Spanish Society of Pneumology and Thoracic Surgery (Sociedad Española de Neumología y Cirugía Torácica/SEPAR).
Conflict of interestsThe authors have no relevant financial or non-financial interests to disclose.