The main purpose of this narrative review is to educate general practitioners about a crucial pleural procedure, namely local anesthetic thoracoscopy (LAT), and to provide established respiratory physicians with an expert opinion-based summary of the literature. This narrative review focuses on the indications, technical aspects and complications of LAT, highlighting its safety and high degree of diagnostic sensitivity for patients who present with an unexplained pleural effusion and have a high pre-test probability of cancer.
Thoracoscopy, a technique for examining the thoracic cavity, has been in use since the 19th century. Hans-Christian Jacobaeus is credited with introducing the method of using a cystoscope to insufflate air into the thoracic cavity and diagnose tuberculous pleural adhesions in 1910.1 However, the French may have been the first to conceptualize the idea of thoracoscopy, with medical texts using the term as early as 1842. Sir Francis Richard Cruise, an Irish endoscopist, used regular direct endoscopic inspection of the pleura in 1865 to monitor the recovery of patients from pleural infections. Together with Samuel Gordon, an Irish internist, Cruise used a modified endoscope created by A.J. Desormeaux. Additionally, in 1882, Carlo Forlanini in Pavia, Italy, used a similar method to induce lung collapse as a treatment for pulmonary tuberculosis.2 Thoracoscopy became an infrequent technique after 1950, especially in the United States.3 Thoracoscopy was primarily utilized during this time to help diagnose pleural effusions and to perform pleurodesis using silver nitrate or talc.4,5 The thoracic cavity was examined using hard bronchoscopes, mediastinoscopes, and specialist fiber-optic rigid scopes.
A semi-rigid scope with a flexible end was originally introduced for thoracic cavity inspection in the late 1990s.6 The shrinking of video cameras and technological advancements were the primary causes of the resurgence of thoracoscopy. With the use of these scopes, the performer and his assistant were able to thoroughly examine the intended space and view the photographs on video monitors. When affixed to a fiber-optic telescope, the video camera might serve as a valuable instrument for prompt examination as well as for instructing and training fellow medical professionals who will be witnessing and supporting the process. For its diagnostic and therapeutic uses in the thoracic cavity, thoracoscopy—once abandoned as a treatment in the management of tuberculous pleural adhesions—has regained prominence and is now often performed by internists and surgeons.
Direct visualization of the pleura enables biopsies to be taken from the parietal pleura and pleural-based abnormalities such as nodules, increasing the diagnostic yield, particularly in malignancy.7 Simultaneously, any fluid can be drained, providing symptomatic relief to the patient, and measures such as talc pleurodesis or indwelling drains can be respectively performed or inserted for fluid management.8
Local anesthetic thoracoscopy (LAT), also known as medical thoracoscopy or pleuroscopy, has grown in popularity among respiratory doctors as a method for diagnosing exudative pleural effusions whose etiology has not yet been established.8,9 The procedure is widely used in the United Kingdom (UK). 39 centers provided this service in 2009; a more recent survey conducted in 2018 by De Fonseka et al. discovered that 49 centers provided LAT on a regular basis to patients.10 The survey showed that both rigid and semi-rigid thoracoscopes were available, and there was wide variation in the pre-procedure use of antibiotics, the use of thoracic ultrasound (TUS), the decision to proceed when there was little fluid present, and the use of midazolam and/or fentanyl for sedation and pain relief, as well as the performance of combined procedures.
In the paragraphs that follow, some of those points will be covered in more detail. These results are not exclusive to the UK; a survey conducted in India revealed a similar variation in practice.11 Since most current practice is based on the now-outdated British Thoracic Society 2023 guidelines,12 narrative reviews like this one can be a useful resource in the absence of more recent, up-to-date publications. Image-guided pleural biopsies (directed by TUS or computed tomography [CT]), which have reported sensitivities between 84 and 93 percent, are an alternative to LAT.13 However, they lack real-time tumor visualization and do not help the patient if the offending effusion is not drained. The only study that directly compares CT guided biopsy and LAT found no statistically significant difference in the diagnostic rates between the two groups (CT-guided biopsy 87.5 percent vs. 94.1 percent for thoracoscopy).14
LAT can be used to treat malignant pleural effusions, pleural infections, and pneumothorax. We will particularly focus on LAT in malignant pleural illness. Excellent articles have been written about the rising incidence of malignant pleural disease,15,16 and LAT is a critical component of the diagnostic pathway.8
IndicationsMalignant pleural effusionLAT is mostly used to verify the presence of a malignant disease in the pleural space. It has a 92.6 percent diagnostic sensitivity for malignant pleural illness, according to several case series.8,9 Obtaining pleural fluid cytology is the initial step in the diagnosis of pleural space malignancy, which frequently manifests as a pleural effusion. The likelihood of a positive cytological result varies by type of cancer, with lung adenocarcinoma and ovarian cancer having the highest yield17–19 compared to other malignancies like pleural mesothelioma (PM) or squamous cell lung cancer. Pleural fluid cytology only provides a 6–32 percent diagnostic yield in PM, and a parietal pleura biopsy is frequently needed to fully characterize the tumor.17–19 There is strong support for the direct use of LAT when the chance of MPM is high, based on clinical and radiological characteristics, and fluid cytology is negative or inconclusive.20 A direct LAT method can accomplish one of the unwritten principles of pleural disease, which is “how to obtain a diagnosis and prevent recurrence for my patient in the least feasible steps.” The approach also gives simultaneous access to therapeutic interventions, such as the delivery of talc for pleurodesis.8–10
Pleural infectionLAT has been used to treat pleural infections, pneumothoraxes, and perform lung biopsies and sympathectomies.9 Although it is asserted that the importance of formal surgery in pleural disease is decreasing.21 Only pleural infections at stages 1 and 2, or the initial two stages of pleural infection (simple exudate, fibrinopurulent stage, and then the third, an organizing stage with pleural peel formation), are susceptible to LAT.21–23 During the procedure, adhesions are broken, fluid is drained, and a chest tube is inserted. Although it is not a novel idea, it has not yet been investigated through multi-center randomized trials, nor has any fresh advice on the subject been published.24,25 Eight studies, all of which were case series or retrospective observational studies, but two which were multi-center, were included in a recent systematic review on the efficacy of LAT in pleural infection.26 Although LAT had a pooled treatment success rate of 85% (95 percent CI 80.0–90.0 percent;) when used as a first-line intervention or in cases where intrapleural therapy or regular tube drainage had failed, the study designs were suboptimal, and there was a high risk of bias. As a result, it is challenging to recommend LAT for pleural infection. When compared to chest tube drainage and intrapleural enzyme therapy, a small randomized clinical research with 32 patients suggested that LAT might shorten the length of stay,17 although these results are not yet generalizable.23,27,28
The diagnostic yield of microbiological culture from pleural specimens fluids collected during the procedure was also 12.5 percent higher in individuals who received LAT27 in comparison to chest tube dreinage. A recent multi-center UK study called “Studying Pleuroscopy in Routine Pleural Infection Treatment” (SPIRIT) will hopefully yield more information about the function of LAT in pleural infection.24 As a side note, LAT has a diagnostic sensitivity of up to 100% in tuberculous pleural illness, but its usage is restricted in many nations due to its high resource requirements.29 In regions with a high prevalence of tuberculosis, a combination of pleural fluid adenosine deaminase, differential cell count, and closed pleural biopsy is equally effective.29
PneumothoraxThe primary goal of surgery in patients with pneumothorax is to prevent recurrence; nevertheless, the whole range of possible operations is outside the scope of this article. These are often carried out using an open thoracotomy technique or video-assisted thoracoscopy (VATS).30 LAT for pneumothorax is not a novel idea either. The biggest case series of 124 pneumothorax patients received talc poudrage pleurodesis with electrocoagulation of blebs/bullae under LAT with an average operating time of 15min.31 Four (4%) patients needed further surgery. However, is the preferred operative approach in pneumothorax. A skilled thoracoscopist can also conduct sympathectomy9 and lung biopsy, though both procedures have almost entirely been replaced by VATS.32
PatientsPrior to LAT, careful patient selection is necessary. It's crucial to have a thorough understanding of the disease process, including any prior occupational exposure to carcinogens like asbestos and malignant diseases. A World Health Organization Performance Status of 2 or above is advised since a baseline functional assessment is helpful to determine eligibility for the operation as well as potential treatment.33,34 The medical comorbidities of the patient may reveal crucial details about the patient's risk factors for the treatment, such as drug intolerances and allergies. Clopidogrel, prasugrel, and ticagrelor should be stopped 5 days before the operation, and ticagrelor should be stopped 7 days before the procedure. There is no reason to discontinue taking aspirin. Therapeutic low molecular weight heparin should be withheld for 24h, warfarin for 5 days, and direct oral anticoagulants typically for 48h; however, dagibatran may need to be stopped 4 days before the procedure if the patient's creatinine clearance is less than 50mL/min/1.73m2. Additionally, the international normalized ratio should be less than 1.5 and platelet counts should be greater than 50,000/μL.8,12,33,34 Notably, in our opinion, platelet activity is also crucial, and advise from hematology may be sought before the procedure in a thrombocytopenic patient, even though platelet count is above the safe established threshold.12,34 To determine the technical suitability and optimal point for thoracoscopy, chest radiographs, CT scans and, particularly, TUS should be obtained before to LAT. Although neither imaging modality is 100% accurate in excluding adhesions, CT and TUS are crucial for detecting pleural thickening, pleural enhancement, and adhesions that may complicate the treatment.35,36 Only a few conditions represent an absolute contraindication to LAT, such as advanced empyema, especially when it has considerable adhesions that might made it unsafe to place the thoracoscope, and the fusion of the visceral and parietal pleura in presumed mesothelioma. Hemodynamic instability, severe hypoxemia, severe coagulopathies, refractory cough, and medication hypersensitivity are relative contraindications.8,9 A thorough assessment of the patient as well as the indications and contraindications to the procedure is required because it is an invasive procedure that the chest physician should only use when other, simpler methods fail to yield a diagnosis or when less invasive therapeutic measures are not available or less promising.37
A fundamental part of the decision-making process for medical thoracoscopy is a thorough examination of the patient's clinical history. Whether the illness in question is acute or chronic, its evolution can provide important information about its nature and course. When deciding if medical thoracoscopy is appropriate for use as a diagnostic or therapeutic procedure, this information becomes crucial. For example, different strategies may be needed for chronic illnesses than for acute presentations, and a thorough clinical history helps to customize the treatment to the unique features of the pleural effusion.38
An extensive physical examination yields information that is useful in evaluating pleural effusions. Clinical indicators of pleural pathology include diaphoresis, diminished vocal resonance, and increased vocal fremitus. These physical indicators help to detect and characterize pleural effusions, which is important information for making decisions. A multifaceted approach is ensured by including physical examination findings into the patient evaluation technique. This allows for a more nuanced comprehension of the clinical presentation and guides subsequent therapeutic or diagnostic actions.38
Moreover, Medical thoracoscopy success and safety are greatly dependent on the proficiency of the thoracoscopist. It is imperative to have sufficient training in both technical and cognitive abilities. By ensuring that the thoracoscopist can accurately navigate the procedure, this training helps to minimize the possibility of difficulties. The total effectiveness of the treatment and the outcomes for the patient are enhanced by a well-trained thoracoscopist, highlighting the significance of continuing education and training in this specialized field.9 In order to improve procedural knowledge and instrumentation familiarity, at least 20 procedures should be performed. This cutoff point illustrates how crucial practical experience is to maximizing a thoracoscopist's abilities. Reaching this level of proficiency guarantees competency, which enhances medical thoracoscopy's safety and efficacy. The focus on a minimal number of procedures highlights how important it is to have real-world experience in order to fully grasp the complexities of this diagnostic and treatment approach.9
Anesthesia and techniquesSedationAccording to the BTS 2023 recommendation,12 midazolam and fentanyl are typically used in combination to sedate patients. Propofol is sometimes used as an alternative to midazolam for sedation.39 When compared to midazolam, propofol had a higher incidence of hypoxemia and hypotension, according to a non-inferiority trial by Grendelmeier et al.,39 but Tschopp et al.40 showed that bispectral index guided propofol was a safe sedation method that facilitated early discharge after the procedure. Other analgesic techniques have been used,41–43 such as intercostal nerve block, erector spinae plane block, or intrapleural lidocaine using semi-rigid thoracoscopes. There is no standardized sedative technique.
ProcedureThere are no requirements for the hospital other than medical staff (typically a respiratory physician with an interest in pleural disease or an interventional respiratory physician) and nursing staff must be trained in the procedure and be able to handle any complication that might arise or refer to a local or regional cardiothoracic unit if urgent surgical intervention is required. As described by Bodtger et al.44 Medical thoracoscopy can be performed in a surgical theater or an interventional suite. The patient will lie in the lateral decubitus position with the side of the pleural effusion to be examined facing upwards after being sedated and being continuously monitored (blood pressure, oxygen saturation, and pulse rate). A TUS is useful to select a puncture site, and local anesthetic will be injected there. The parietal pleura will then be punctured using a Boutin needle that has both blunt and sharp ends after a minor incision is made with a scalpel.45 Then, it only takes the patient 10–20 spontaneous breaths to cause a pneumothorax, causing the lung to “peel away” from the parietal pleura. A path is then made from the skin to the pleura using tiny forceps, and a 7-millimeter (mm) trocar is then inserted through the tract. To allow for inspection of the pleura and lung with either zero- or fifty-degree scopes and then biopsy any lesions deemed appropriate, fluid can be removed through the trocar using standard suction tubing (due to equalization of pleural and atmospheric pressure during induction of the above pneumothorax, large amounts of pleural fluid can be removed without the risk of re-expansion pulmonary edema). A large bore drain or an indwelling pleural catheter can then be placed after talc poudrage. Pulmonologists often utilize a rigid thoracoscope with a single port10; however, some execute LAT with a semi-rigid thoracoscope that resembles the more popular flexible video bronchoscope.46–49 The rigid thoracoscope's benefits include its capacity for effective sampling and therapeutic interventions such talc poudrage and adhesion breakup. The semi-rigid thoracoscope provides a larger field of vision because of its flexibility, but it occasionally needs a second entrance port for better visualization. It also acquires smaller tissue biopsies. This was demonstrated by Rozman et al.,48 who found that rigid forceps produced larger samples (24.7mm2) than semi-rigid ones (11.7mm2). Diagnostic precision and safety profile are comparable between the two types of thoracoscopes.38–42
In a recent meta-analysis by Manoharan and Argaez,50 they analyze the diagnostic yield between rigid and semi-rigid thoracoscopy was found to be overlapping, the costs of medical thoracoscopy are lower than those of VATS. They made up this meta-analysis including studies that compared the costs of semi-rigid thoracoscopy to VATS, and the diagnostic yield. In particular, the diagnostic accuracy between these studies is from 91% (in one study, for rigid thoracoscopy) to 100%, with sensitivity between 85% and 100% and specificity between 94% and 100%. The positive predictive value was from 99 to 100%.
According to a high-quality comprehensive study, individuals with pleural effusions of unclear cause may find MT helpful in ruling out a diagnosis if the pooled negative probability ratio is less than 0.1
There were not many significant procedural complications among the included systematic reviews, diagnostic investigations, randomized and non-randomized research that detailed adverse events related to MT. Moreover, the mean procedure-related cost of semi-rigid thoracoscopy was $2815 Canadian dollars (95% CI $2010 to $3620) according to an economic review, while the VATS was $7962 Canadian dollars (95% CI $7134 to $8790). P-value<0.001 in individuals with pleural effusions that go undetected. The lengthier hospital stay associated with VATS may have contributed to part of the treatment cost differential, as all VATS procedures were performed in the hospital, while 68% of semi-rigid thoracoscopy procedures were performed as outpatient procedures.51
Fig. 1 provides a summary of the various step required to perform a LAT.
Comparison of techniquesThe most widespread techniques for the approach to the pleural spill are the thoracentesis, the percutaneous pleural biopsy and the association of the medical thoracic scan with the analysis of pleural fluid.52
Thoracentesis is a diagnostic procedure that helps to confirm the existence of pleural effusion by removing fluid from the pleural cavity. Although it offers validation, it can require enhancement in pinpointing the exact origin of the effusion. The shortcomings of thoracentesis highlight the need for additional diagnostic methods in order to identify the underlying cause.38
A method to a conclusive diagnosis is provided by percutaneous pleural biopsy, which uses a needle to take a sample of the pleural tissue. But it might not be entirely accurate, particularly when there is malignant pleural illness. With reported results as high as 95% for malignant pleural illness, medical thoracoscopy becomes a more accurate alternative when sensitivity and specificity become critical.53
A thorough diagnostic technique is presented by combining pleural fluid analysis (adenosine deaminase; ADA) and protein levels with other procedures (closed needle biopsy, histology, and culture). This method exhibits great specificity (100%) and sensitivity (about 93%), especially in areas where tuberculosis (TB) is prevalent. It offers accurate and effective diagnostic results at a lower cost than medical thoracoscopy.38
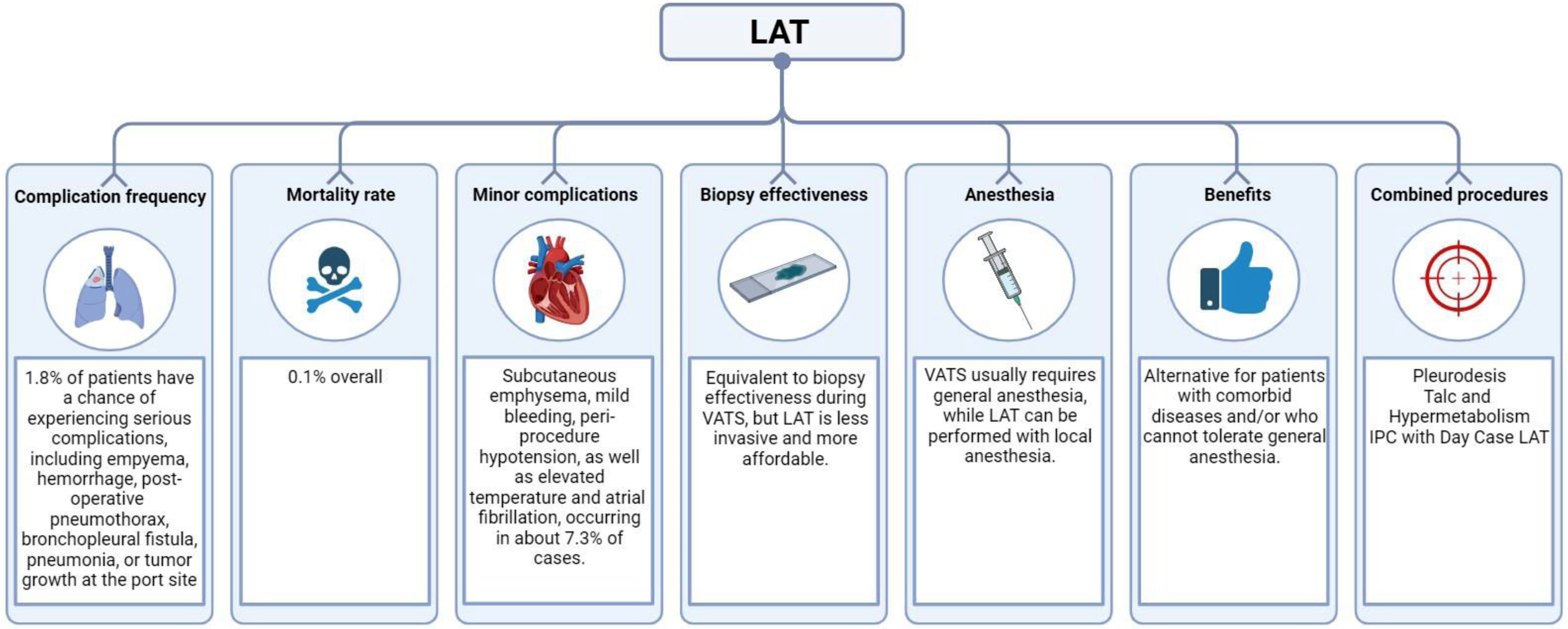
ComplicationsThe British Thoracic Society's guidelines8 characterize LAT as being a very safe technique. In 1.8 percent of patients, there is a chance of experiencing a serious complication, such as empyema, hemorrhage, post-operative pneumothorax, bronchopleural fistula, pneumonia, or tumor growth at the entry port site.8,29 The mortality rate is 0.1 percent overall.8,33,34 Although non-graded talc was being used at the time, fatality cases came from older series and linked to talc-induced respiratory failure brought on by acute respiratory distress syndrome (ARDS). No new cases of ARDS have been noted since graded talc became widely used. Some facilities frequently prescribe antibiotic prophylaxis to prevent pleural space or skin infections.54 When Dhooria et al. examined the impact of patients receiving a single dose of antibiotic on infection rates,55 they found no substantial difference. Subcutaneous emphysema, mild bleeding, peri-procedure hypotension, as well as elevated temperature and atrial fibrillation, are examples of minor problems (overall frequency 7.3%).8,12,33,34,54
Typical complicationsA common consequence that affects about 32.74% of patients undergoing medical thoracoscopy is post-thoracoscopic discomfort. This pain can interfere with the patient's comfort and recuperation and is a normal side effect of the surgery. Anesthetics and other pain management techniques may be used as management solutions for pain following a thoracoscopic procedure. Because pain is so common, it's critical to implement proactive pain management strategies in order to improve patient satisfaction and the procedure as a whole.56
Transient air leaks, which occur in about 11.61% of cases, are a manageable complication associated with medical thoracoscopy. They are typically addressed effectively with conservative measures and, despite causing some postoperative discomfort, usually do not pose a significant threat to the patient's health due to their transient nature. Successful management of this complication is often achieved through adequate patient education and monitoring.56 Fever is another complication seen in 3.9% of cases, indicating the inflammatory response triggered by medical thoracoscopy. While it's common, it's crucial to monitor it closely to distinguish between an expected postoperative fever and potential systemic infections. Early detection and proper management help reduce the fever's impact on patient recovery.56 Wound infections, though rare, can occur as complications of medical thoracoscopy. Preventive measures include adhering to aseptic techniques during the procedure and providing meticulous postoperative care. Quick identification and intervention in cases of wound infection are vital to minimize further complications and ensure the best patient outcomes.9 Empyema, although rare, is a serious complication that emphasizes the need for careful postoperative monitoring. It's characterized by pus accumulation in the pleural space and requires immediate diagnosis and treatment. Preventive strategies include rigorous infection control practices and close monitoring for any signs of postoperative infection.9 Subcutaneous emphysema, which occurs in 0.8% of cases, involves the presence of air in the subcutaneous tissues. It's usually self-limiting but underscores the importance of monitoring for any signs of respiratory distress. Educating patients about expected postoperative changes can help them understand and feel reassured about this temporary complication.57 Lastly, re-expansion pulmonary edema is a rare but severe complication, especially in patients with pre-existing respiratory conditions. It highlights the importance of careful patient selection and consideration of individual risk factors. Preoperative assessment and optimization of respiratory status can help reduce the risk of this complication.9
A holistic strategy to reduce complications includes meticulous patient selection, guaranteeing that thoracoscopists possess sufficient training, and following recognized surgical methods and guidelines. Prompt detection and suitable handling of complications enhance the overall safety and efficacy of medical thoracoscopy, leading to positive results for the majority of patients.38,58
Major complicationDuring thoracoscopy, lung lacerations require immediate and focused intervention. The first step is to apply pressure to the bleeding point using the surrounding lung tissue, effectively controlling, and stopping the bleeding. An hemostatic agent can be used to further stop the bleeding. It's essential to manage lung lacerations with a swift and coordinated response to minimize potential harm and ensure patient safety.59
Pulmonary complications such as hypoxemia, hypercapnia, re-expansion pulmonary edema, and atelectasis call for a comprehensive strategy in line with established medical protocols and guidelines. Management tactics might include interventions like supplemental oxygen therapy, mechanical ventilation if necessary, and careful monitoring of respiratory parameters. Customizing the approach to the specific pulmonary complication is crucial for optimizing patient outcomes and preventing further respiratory compromise.38,60
In situations where hospital-acquired infections (HAIs) are a risk, immediate medical treatment is initiated for control. Preventive measures might involve administering a single dose of intravenous broad-spectrum antibiotics during the procedure. This proactive approach reduces the risk of infections related to the thoracoscopic intervention. The careful use of antibiotics is in line with infection control principles, preventing HAIs and enhancing patient safety.58
Preventing complications in thoracoscopy is of utmost importance and starts with strictly adhering to established operational standards. This includes careful attention to patient positioning to ensure optimal access and visibility during the procedure. Utilizing available imaging techniques can increase precision and reduce the risk of adverse events. By prioritizing preventive measures and following standardized protocols, healthcare professionals can significantly decrease the chances of complications during thoracoscopy. This proactive approach is in line with the principles of patient safety and procedural excellence.61
LAT vs. VATSIt should be noted that in the diagnosis of malignancy, biopsy performed during LAT is just as effective as biopsy performed during VATS, the latter being typically carried out under general anesthesia by thoracic surgeons, although local anesthetic approaches have been described.62,63 LAT provides an alternative for patients with comorbid diseases and/or who are unable to endure general (and single lung ventilation) anesthesia because it is less intrusive and more affordable than VATS.62,63
Increasing diagnostic output of LATAlthough the diagnostic yield of LAT is very high, other procedures (VATS or image-guided biopsies) are occasionally needed for the small percentage of patients in whom a malignant diagnosis is suspected (those with high pre-test probability, for example, with red flag symptoms and pleural nodules and masses on imaging) and whose biopsy is interpreted as benign (inferring a false negative biopsy).54,62–64 According to anecdotal evidence, this significantly increases patient anxiety and slows down any viable therapy pathway. Additional factors, like a delay in pathology processing, can worsen these effects.64 With reported sensitivities of 36–51% and specificities between 88 and 100%, pleural masses, nodules, thickness, and irregularity on concurrent radiological imaging are linked with high pre-test probability for cancer at LAT.65,66
False negative results are frequently attributed to “difficult” LAT, where the amount of fluid may be minimal, the lung may not “go down” during pneumothorax induction resulting in limited views, there may be significant loculations or adhesions limiting access to the parietal pleura, or “deep” (incorporating adipose and muscle tissue) parietal lining biopsies were not performed or were not practical.67
The difference between a genuine false negative biopsy and pathological evidence that would support non-specific pleuritis (NSP), which is characterized by chronic pleural inflammation without a clear benign or malignant origin, must be understood. NSP may be the ultimate diagnosis in up to 30% of exudative pleural effusion cases.67 However, because to the possibility of malignant change, thorough clinical and radiological follow-up is necessary for a minimum of 24 months.67,68
Since the pleura's macroscopic appearance is neither sensitive nor specific, a variety of suggested techniques are provided to increase the diagnostic yield.69 First, despite the challenges in diagnosing some cancers, like mesothelioma, where it may be impossible to distinguish between benign fibrinous material, atypical mesothelial cells, and tumor using rapid onsite evaluation (ROSE), it could be more useful to let the histopathologist perform the evaluation of the specimens at the time of LAT. Although ROSE has not been put to the test in extensive multi-center studies, monocentric observational data indicate that integrating radiological imaging and ROSE findings might improve diagnostic yield.70 However, general applicability is difficult since, for instance, there aren’t enough histopathologists or cytotechnologists in Italy, at least, to make having one present at every LAT procedure.71
Confocal laser endomicroscopy is a fresh, intriguing breakthrough that was just reported initially at the 2020 and 2021 European Respiratory Society Congress.72 This optical imaging device, used during endoscopy, gives real-time in vivo images of tissues and can identify cancerous cells. The biggest case series of 62 patients accurately described benign and malignant pathology, with 100% positive predictive value (ppv) for the “whole chia seed sign” (75 percent ppv for abnormal tissue architecture and 68 percent for dysplastic vessels). Again, more extensive, multi-center research is necessary for the external validation of those conclusions.72 Similar to this, autofluorescence and narrow band imaging (NBI), which illuminate aberrant tissues and permit targeted biopsies, have been proposed; however, their widespread application depends on training and resources.73–75
Combined proceduresIf cancer is highly suspected, pleurodesis is frequently performed.8–10 This is a crucial component of managing pleural malignancy, but if pleurodesis is carried out, further interventional operations may be challenging, and talc may manifest as aberrant hypermetabolic regions on CT or positron emission tomography (PET) scans.76 As demonstrated by Bhatnagar et al.,77 talc poudrage during thoracoscopy had no difference in the rate of pleurodesis at 90 days when compared with talc slurry through a chest tube. Therefore, pleurodesis at thoracoscopy should also be guided by patient-centered management. It could be argued that doing a pleurodesis concurrently with a LAT for fluid control and diagnosis saves the need for a separate procedure, but pleurodesis via LAT shouldn’t be recommended as the best option. In the past few months, there has been a discernible shift toward day case LAT with indwelling pleural catheter (IPC) insertion, which is supported by the pleural guidance published by the British Thoracic Society at the beginning of the COVID-19 pandemic. This shift is evidenced by case series.78 In the UK, a trial is being conducted to compare the benefits of normal care—a big bore drain, pleurodesis, and admission—with day case LAT and IPC insertion (and aggressive following draining via the IPC).79 If the ROSE preparation shows a non-malignant process in a patient with a high index of suspicion for malignancy, another technique (such endobronchial ultrasonography) may be performed concurrently.80 In most centers, this is not typical, and it would again have a huge resource impact. Fig. 2 provides a concise overview of critical aspects related to complication and benefits of LAT.
Future developmentSignificant advancements in imaging techniques, especially magnetic resonance imaging and computed tomography, have greatly improved diagnostic capabilities for pleural effusions. The enhanced image quality provided by these technologies allows healthcare professionals to see pleural abnormalities with increased clarity and precision. These imaging methods are crucial in diagnosing and characterizing pleural effusions, leading to more precise and targeted treatment plans. The integration of these advanced imaging techniques is a key part of modern diagnostic methods, enabling a thorough evaluation of pleural diseases.81,82
In addition, the field of pleural diseases has been transformed by computer-aided diagnostic analysis, which can specifically identify cancer cells in pleural fluid. Deep learning algorithms, trained on CT image data, show promising capabilities in identifying characteristics and diagnostic criteria for pleural effusion diagnosis. This technological breakthrough has the potential to enhance the accuracy and efficiency of diagnostic procedures, offering valuable insights that can inform clinical decision-making. The incorporation of computer-aided diagnostic tools signifies a state-of-the-art approach for achieving more accurate and reliable diagnostic results in pleural diseases.81
Future research is crucial to determine the best timing for medical thoracoscopy in managing pleural infections and its potential role in early diagnosis and treatment of pleural diseases.27 The timing of this intervention can significantly affect patient outcomes. Studies in this area can help establish evidence-based guidelines, optimize the use of medical thoracoscopy in clinical practice, and enhance its effectiveness as a diagnostic and therapeutic tool.
Moreover, additional research on the cost-effectiveness of different thoracoscopy techniques, including semi-rigid and rigid thoracoscopy, is essential for improving the diagnostic and therapeutic capabilities of medical thoracoscopy.53,83 Comparative studies can offer valuable insights into the relative pros and cons of these techniques, guiding decision-making for clinicians and healthcare systems. It's important to understand the economic impact of these techniques to optimize resource distribution and ensure the sustainability of medical thoracoscopy in various healthcare environments.
Lastly, ongoing investigation into the latest advances in diagnosing pleural effusion is vital to stay updated with emerging technologies. Continuous research can provide valuable insights into new diagnostic tools and methods, their effect on diagnostic accuracy, and their potential to enhance patient outcomes.84 Staying current with technological advancements ensures that healthcare practitioners can utilize the most effective diagnostic methods in the ever-evolving field of pleural effusion diagnostics.
ConclusionsLAT is a safe treatment with a high degree of diagnostic sensitivity for patients who present with an unexplained pleural effusion and have a high pre-test likelihood of cancer. The aforementioned narrative review is intended to educate general practitioners about a crucial pleural procedure and to provide established respiratory physicians with an expert opinion-based summary of the literature.
FundingNone.
Conflict of interestNothing to declare.