Bronchiectasis, particularly in children, is an increasingly recognised yet neglected chronic lung disorder affecting individuals in both low-to-middle and high-income countries. It has a high disease burden and there is substantial inequity within and between settings. Furthermore, compared with other chronic lung diseases, considerably fewer resources are available for children with bronchiectasis. The need to prevent bronchiectasis and to reduce its burden also synchronously aligns with its high prevalence and economic costs to health services and society.
Like many chronic lung diseases, bronchiectasis often originates early in childhood, highlighting the importance of reducing the disease burden in children. Concerted efforts are therefore needed to improve disease detection, clinical management and equity of care. Modifiable factors in the causal pathways of bronchiectasis, such as preventing severe and recurrent lower respiratory infections should be addressed, whilst also acknowledging the role played by social determinants of health.
Here, we highlight the importance of early recognition/detection and optimal management of bronchiectasis in children, and outline our research, which is attempting to address important clinical knowledge gaps discussed in a recent workshop. The research is grouped under three themes focussing upon primary prevention, improving diagnosis and disease characterisation, and providing better management. Our hope is that others in multiple settings will undertake additional studies in this neglected field to further improve the lives of people with bronchiectasis. We also provide a resource list with links to help inform consumers and healthcare professionals about bronchiectasis and its recognition and management.
Bronchiectasis is a clinical syndrome where the most common symptom is a persistent or recurrent wet/productive cough.1,2 The diagnosis is confirmed objectively by identifying abnormally dilated bronchi with an increased broncho-arterial ratio (BAR) in a chest computed tomography (CT) scan.1,2 Globally, bronchiectasis is said to be the third most common chronic airway disorder,3,4 behind asthma and chronic obstructive pulmonary disease (COPD), and a leading cause of death and disability worldwide in adults.1,5 Indeed, adults with severe COPD may also have bronchiectasis,6 but this diagnosis is difficult to confirm without confirmation by a chest CT scan. Bronchiectasis has multiple aetiologies and affects all age groups and socio-economic classes in both high-income7–10 (HIC) and low-to-middle income countries5,11,12 (LMICs) and, for reasons that are uncertain, rates of disease and reported aetiology vary substantially in different populations.13 However, the burden of disease is greatest in socio-economically disadvantaged communities, including Indigenous populations in HICs.14
Importantly, in children with early, cylindrical bronchiectasis, symptoms can resolve and the abnormal bronchial dilatation may be reversed when an early diagnosis is made and optimal management is instituted.1,5,15,16 Also, there is increasing recognition that periconception, antenatal17 and early childhood factors, including chronic bronchitis (a component of bronchiectasis5), are mediators of future lung disease (e.g. COPD), poorer lung function and premature mortality in adults.18,19 Consequently, focusing upon children is important for reducing adult lung disease19,20 and preventing suboptimal lung function.21 To achieve this goal in bronchiectasis, its profile in children must be raised further to enable its prompt recognition and improved management by healthcare professionals.
Australian and English studies report many adults with bronchiectasis have symptoms from childhood.22,23 In the Australian study of 103 adults with bronchiectasis referred to a single centre, symptoms dating back to childhood were reported in 59% and associated with more severe disease (worse radiographic scores and poorer lung function) and worse prognosis than those whose symptoms began later in life.22 Their key symptom was chronic productive/wet cough and with its longer duration, the poorer the lung function, as reflected in lower forced expiratory volume in 1-second (FEV1) values (r −0.51, p<0.001 in non-smokers).22
Thus, to reduce the global burden of bronchiectasis, we need to concentrate not only upon established bronchiectasis, but also upon the recognition and evidence-based management of earlier symptoms. The most important is the identification of chronic wet/productive cough in children as early intervention may prevent progression, improve long-term outcomes and even reverse bronchial dilatation in those with mild disease.1,2,24 At the same time, the urgency of addressing the social determinants of health is also acknowledged.14
This review resulted from our Australian National Health Medical Research Council (NHMRC) Centre of Research Excellence (CRE) for child bronchiectasis (AusBREATHE) workshop. Here, we highlight the importance of prompt diagnosis and optimal management of bronchiectasis in children. We also discuss interventions exemplified by our research, with our RCTs undertaken in Australia, and in collaboration with others in New Zealand and South-East Asia (Malaysia, Philippines, Timor-Leste), in the hope that others in multiple different settings will undertake studies to address clinical knowledge gaps in this neglected field and improve the lives of people with bronchiectasis.
The need to reduce the global burden of bronchiectasisDespite the increased recognition of bronchiectasis1,5 and its known substantial morbidity5 and mortality,25 it remains a neglected lung disease26 with large unmet needs, especially in children.27,28 The importance of reducing the global burden of childhood bronchiectasis includes its disease burden, health inequity, and its links with future lung health.
Globally, the prevalence of bronchiectasis (67–1100 per 100,000 population29) is far higher than that of cystic fibrosis (CF) (1 in 3000 Caucasians, 1 in 4000–10,000 Latin Americans30).1 In addition to the prevalence burden, bronchiectasis also has a high patient-care burden,31 and high individual health needs28 and economic cost.5,27,32 In a systematic review, the annual healthcare cost for adults ranged in United States dollars (USD, standardised to 2021 USD) from USD3579 to 82,545 and indirect costs (e.g. lost income) ranged from USD1311 to 2898.32 The annual aggregate healthcare cost estimates varied from USD101.60 million in Singapore to USD14.68 billion in the United States, while in Australian children it was USD17.77 million.32 Respiratory exacerbations and hospitalisations33 drive these costs. In a prospective, multicentre cohort study of 85 children with bronchiectasis from Australia and New Zealand,31 mean exacerbations per child-year was 3.3 (standard deviation=2.2) with 11.4% of 264 episodes requiring hospitalisation. Absences from school or childcare due to bronchiectasis were 24.9 children per 100 child-months and quality-of-life (QoL) scores were negatively associated with cough severity.31
Reducing bronchiectasis is also important from a health equity perspective. On average, Australian Indigenous people with bronchiectasis die ∼22-years earlier than other Australians with bronchiectasis.25,34 This gap is substantially larger than for cardiovascular disease (where the gap is 9-years),25 yet bronchiectasis receives relatively little attention and resources, both clinically and in research. A large equity gap also exists within settings.35 A New Zealand study found that despite adolescents with bronchiectasis having significantly worse lung function than those with CF, they received less formalised transfer to adult services, and had fewer clinic appointments and resources available to them than patients with CF.36 An Australian specialist paediatric hospital study showed that children/adolescents with bronchiectasis have significantly and substantially poorer lung function than age-matched children/adolescents with CF (mean FEV1=78.6%predicted [standard deviation=20.5] vs 94.5%predicted [14.7] respectively), and received a lower standard of care (fewer clinic visits, physiotherapist reviews and lung function tests).35 These studies demonstrate the urgent need to improve detection, clinical management and equity of care so as to reduce the overall burden of bronchiectasis.
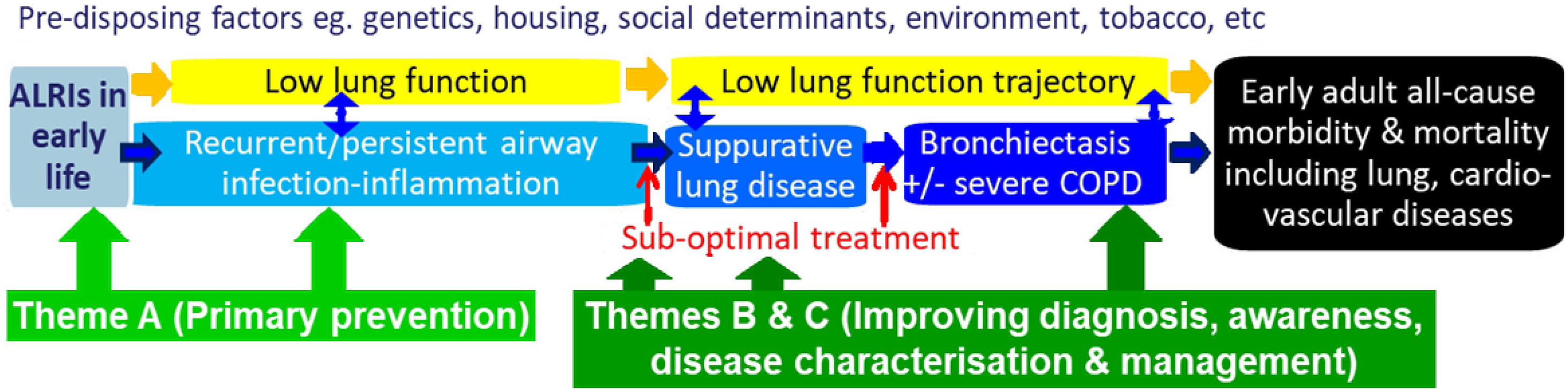
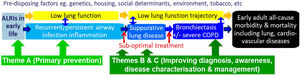
Our paradigm, CRE framework and outcomesOur previous CRE37 was framed around a paradigm, which, if symptoms were left untreated, involved a continuous clinical spectrum linking protracted bacterial bronchitis (PBB) with initially reversible and then irreversible bronchiectasis.38 Subsequently, further data have consolidated this paradigm1,39 and it is now accepted globally.1,5,40,41 In our current CRE, we have expanded the paradigm (Fig. 1) to include modifiable risk factors of bronchiectasis. A focus on these antecedents is important, not only to reduce the burden of bronchiectasis, but also other chronic lung diseases and suboptimal lung development.
Our current paradigm that frames the work and studies underpinning our Centre of Research Excellence (AusBREATHE). The three themes address different parts of the paradigm, which are explained further in the text. The paradigm is framed around the notion that primary prevention of bronchiectasis is possible, and the knowledge that early detection of causal conditions substantially reduces the risk of developing bronchiectasis by the early initiation of treatment and optimal care. ALRIs, lower respiratory infections.
Addressing the social determinants of health is a priority, but solving clinical issues in children is equally important given its impact upon lifetime health.18,19 Indeed, ignoring the clinical actions until social issues improve risks further disparity as this may take many years. Furthermore, which social interventions have most health impact is uncertain. A systematic overview of interventions addressing the social determinants of health found a striking lack of reliable evaluations.42 Therefore biomedical interventions and advances in healthcare remain essential and these require the best evidence available for preventing and managing illnesses.
To reduce the global burden of bronchiectasis, we need effective and feasible strategies for both primary and secondary prevention that will: (i) optimise early lung health and ameliorate illnesses impacting upon future lung function (e.g. acute lower respiratory infections [ALRIs]21); (ii) improve clinical outcomes of children with chronic cough (the most common symptom of lower airway infection and lung disease, including bronchiectasis1,5,43); and (iii) enhance outcomes of children with established (irreversible) bronchiectasis. As outlined in Fig. 1 and Table 1, our scientific strategies are based upon these themes. They are framed around the notion that primary prevention (Theme A) of bronchiectasis is possible and, its early detection (Theme B) (including causal conditions e.g. hypogammaglobulinemia) substantially reduces the risk of developing established bronchiectasis by promptly initiating optimal care. Many factors (yellow box, Fig. 1) influence progression of the illness and represent potential intervention points. Modifying factors affecting the prognosis of bronchiectasis require better clinical management and guideline implementation (Theme C).
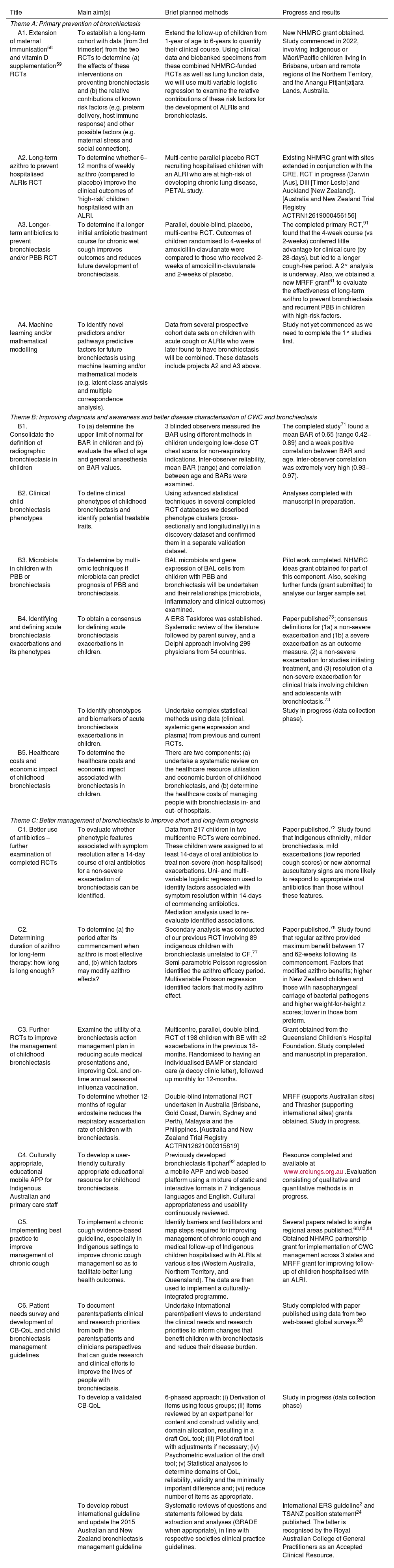
Planned and current studies within AusBREATHE (Australian Bronchiectasis Centre of Research Excellence) that seek to reduce the burden of bronchiectasis in children.
| Title | Main aim(s) | Brief planned methods | Progress and results |
|---|---|---|---|
| Theme A: Primary prevention of bronchiectasis | |||
| A1. Extension of maternal immunisation58 and vitamin D supplementation59 RCTs | To establish a long-term cohort with data (from 3rd trimester) from the two RCTs to determine (a) the effects of these interventions on preventing bronchiectasis and (b) the relative contributions of known risk factors (e.g. preterm delivery, host immune response) and other possible factors (e.g. maternal stress and social connection). | Extend the follow-up of children from 1-year of age to 6-years to quantify their clinical course. Using clinical data and biobanked specimens from these combined NHMRC-funded RCTs as well as lung function data, we will use multi-variable logistic regression to examine the relative contributions of these risk factors for the development of ALRIs and bronchiectasis. | New NHMRC grant obtained. Study commenced in 2022, involving Indigenous or Māori/Pacific children living in Brisbane, urban and remote regions of the Northern Territory, and the Anangu Pitjantjatjara Lands, Australia. |
| A2. Long-term azithro to prevent hospitalised ALRIs RCT | To determine whether 6–12 months of weekly azithro (compared to placebo) improve the clinical outcomes of ‘high-risk’ children hospitalised with an ALRI. | Multi-centre parallel placebo RCT recruiting hospitalised children with an ALRI who are at high-risk of developing chronic lung disease, PETAL study. | Existing NHMRC grant with sites extended in conjunction with the CRE. RCT in progress (Darwin [Aus], Dili [Timor-Leste] and Auckland [New Zealand]).[Australia and New Zealand Trial Registry ACTRN12619000456156] |
| A3. Longer-term antibiotics to prevent bronchiectasis and/or PBB RCT | To determine if a longer initial antibiotic treatment course for chronic wet cough improves outcomes and reduces future development of bronchiectasis. | Parallel, double-blind, placebo, multi-centre RCT. Outcomes of children randomised to 4-weeks of amoxicillin-clavulanate were compared to those who received 2-weeks of amoxicillin-clavulanate and 2-weeks of placebo. | The completed primary RCT,91 found that the 4-week course (vs 2-weeks) conferred little advantage for clinical cure (by 28-days), but led to a longer cough-free period. A 2° analysis is underway. Also, we obtained a new MRFF grant61 to evaluate the effectiveness of long-term azithro to prevent bronchiectasis and recurrent PBB in children with high-risk factors. |
| A4. Machine learning and/or mathematical modelling | To identify novel predictors and/or pathways predictive factors for future bronchiectasis using machine learning and/or mathematical models (e.g. latent class analysis and multiple correspondence analysis). | Data from several prospective cohort data sets on children with acute cough or ALRIs who were later found to have bronchiectasis will be combined. These datasets include projects A2 and A3 above. | Study not yet commenced as we need to complete the 1° studies first. |
| Theme B: Improving diagnosis and awareness and better disease characterisation of CWC and bronchiectasis | |||
| B1. Consolidate the definition of radiographic bronchiectasis in children | To (a) determine the upper limit of normal for BAR in children and (b) evaluate the effect of age and general anaesthesia on BAR values. | 3 blinded observers measured the BAR using different methods in children undergoing low-dose CT chest scans for non-respiratory indications. Inter-observer reliability, mean BAR (range) and correlation between age and BARs were examined. | The completed study71 found a mean BAR of 0.65 (range 0.42–0.89) and a weak positive correlation between BAR and age. Inter-observer correlation was extremely very high (0.93–0.97). |
| B2. Clinical child bronchiectasis phenotypes | To define clinical phenotypes of childhood bronchiectasis and identify potential treatable traits. | Using advanced statistical techniques in several completed RCT databases we described phenotype clusters (cross-sectionally and longitudinally) in a discovery dataset and confirmed them in a separate validation dataset. | Analyses completed with manuscript in preparation. |
| B3. Microbiota in children with PBB or bronchiectasis | To determine by multi-omic techniques if microbiota can predict prognosis of PBB and bronchiectasis. | BAL microbiota and gene expression of BAL cells from children with PBB and bronchiectasis will be undertaken and their relationships (microbiota, inflammatory and clinical outcomes) examined. | Pilot work completed. NHMRC Ideas grant obtained for part of this component. Also, seeking further funds (grant submitted) to analyse our larger sample set. |
| B4. Identifying and defining acute bronchiectasis exacerbations and its phenotypes | To obtain a consensus for defining acute bronchiectasis exacerbations in children. | A ERS Taskforce was established. Systematic review of the literature followed by parent survey, and a Delphi approach involving 299 physicians from 54 countries. | Paper published73; consensus definitions for (1a) a non-severe exacerbation and (1b) a severe exacerbation as an outcome measure, (2) a non-severe exacerbation for studies initiating treatment, and (3) resolution of a non-severe exacerbation for clinical trials involving children and adolescents with bronchiectasis.73 |
| To identify phenotypes and biomarkers of acute bronchiectasis exacerbations in children. | Undertake complex statistical methods using data (clinical, systemic gene expression and plasma) from previous and current RCTs. | Study in progress (data collection phase). | |
| B5. Healthcare costs and economic impact of childhood bronchiectasis | To determine the healthcare costs and economic impact associated with bronchiectasis in children. | There are two components: (a) undertake a systematic review on the healthcare resource utilisation and economic burden of childhood bronchiectasis, and (b) determine the healthcare costs of managing people with bronchiectasis in- and out- of hospitals. | |
| Theme C: Better management of bronchiectasis to improve short and long-term prognosis | |||
| C1. Better use of antibiotics – further examination of completed RCTs | To evaluate whether phenotypic features associated with symptom resolution after a 14-day course of oral antibiotics for a non-severe exacerbation of bronchiectasis can be identified. | Data from 217 children in two multicentre RCTs were combined. These children were assigned to at least 14-days of oral antibiotics to treat non-severe (non-hospitalised) exacerbations. Uni- and multi-variable logistic regression used to identify factors associated with symptom resolution within 14-days of commencing antibiotics. Mediation analysis used to re-evaluate identified associations. | Paper published.72 Study found that Indigenous ethnicity, milder bronchiectasis, mild exacerbations (low reported cough scores) or new abnormal auscultatory signs are more likely to respond to appropriate oral antibiotics than those without these features. |
| C2. Determining duration of azithro for long-term therapy: how long is long enough? | To determine (a) the period after its commencement when azithro is most effective and, (b) which factors may modify azithro effects? | Secondary analysis was conducted of our previous RCT involving 89 indigenous children with bronchiectasis unrelated to CF.77 Semi-parametric Poisson regression identified the azithro efficacy period. Multivariable Poisson regression identified factors that modify azithro effect. | Paper published.78 Study found that regular azithro provided maximum benefit between 17 and 62-weeks following its commencement. Factors that modified azithro benefits; higher in New Zealand children and those with nasopharyngeal carriage of bacterial pathogens and higher weight-for-height z scores; lower in those born preterm. |
| C3. Further RCTs to improve the management of childhood bronchiectasis | Examine the utility of a bronchiectasis action management plan in reducing acute medical presentations and, improving QoL and on-time annual seasonal influenza vaccination. | Multicentre, parallel, double-blind, RCT of 198 children with BE with ≥2 exacerbations in the previous 18-months. Randomised to having an individualised BAMP or standard care (a decoy clinic letter), followed up monthly for 12-months. | Grant obtained from the Queensland Children's Hospital Foundation. Study completed and manuscript in preparation. |
| To determine whether 12-months of regular erdosteine reduces the respiratory exacerbation rate of children with bronchiectasis. | Double-blind international RCT undertaken in Australia (Brisbane, Gold Coast, Darwin, Sydney and Perth), Malaysia and the Philippines. [Australia and New Zealand Trial Registry ACTRN12621000315819] | MRFF (supports Australian sites) and Thrasher (supporting international sites) grants obtained. Study in progress. | |
| C4. Culturally appropriate, educational mobile APP for Indigenous Australian and primary care staff | To develop a user-friendly culturally appropriate educational resource for childhood bronchiectasis. | Previously developed bronchiectasis flipchart92 adapted to a mobile APP and web-based platform using a mixture of static and interactive formats in 7 Indigenous languages and English. Cultural appropriateness and usability continuously reviewed. | Resource completed and available at www.crelungs.org.au.Evaluation consisting of qualitative and quantitative methods is in progress. |
| C5. Implementing best practice to improve management of chronic cough | To implement a chronic cough evidence-based guideline, especially in Indigenous settings to improve chronic cough management so as to facilitate better lung health outcomes. | Identify barriers and facilitators and map steps required for improving management of chronic cough and medical follow-up of Indigenous children hospitalised with ALRIs at various sites (Western Australia, Northern Territory, and Queensland). The data are then used to implement a culturally-integrated programme. | Several papers related to single regional areas published.68,83,84 Obtained NHMRC partnership grant for implementation of CWC management across 3 states and MRFF grant for improving follow-up of children hospitalised with an ALRI. |
| C6. Patient needs survey and development of CB-QoL and child bronchiectasis management guidelines | To document parents/patients clinical and research priorities from both the parents/patients and clinicians perspectives that can guide research and clinical efforts to improve the lives of people with bronchiectasis. | Undertake international parent/patient views to understand the clinical needs and research priorities to inform changes that benefit children with bronchiectasis and reduce their disease burden. | Study completed with paper published using data from two web-based global surveys.28 |
| To develop a validated CB-QoL | 6-phased approach: (i) Derivation of items using focus groups; (ii) Items reviewed by an expert panel for content and construct validity and, domain allocation, resulting in a draft QoL tool; (iii) Pilot draft tool with adjustments if necessary; (iv) Psychometric evaluation of the draft tool; (v) Statistical analyses to determine domains of QoL, reliability, validity and the minimally important difference and; (vi) reduce number of items as appropriate. | Study in progress (data collection phase) | |
| To develop robust international guideline and update the 2015 Australian and New Zealand bronchiectasis management guideline | Systematic reviews of questions and statements followed by data extraction and analyses (GRADE when appropriate), in line with respective societies clinical practice guidelines. | International ERS guideline2 and TSANZ position statement24 published. The latter is recognised by the Royal Australian College of General Practitioners as an Accepted Clinical Resource. | |
ABR=Australian Bronchiectasis Registry; ALRI=acute lower respiratory infection; APP=application; Azithro=azithromycin; BAL=broncho-alveolar lavage; BAMP=bronchiectasis action management plan; BAR-broncho-arterial ratio; CB-QoL=Children's Bronchiectasis Quality-of-Life; CF=cystic fibrosis; CT=computed tomography; CWC=chronic wet cough; ERS=European Respiratory Society; MRFF=Medical Research Futures Fund; NHMRC=National Health and Medical Council; PBB=protracted bacterial bronchitis; QoL=Quality-of-Life; RCT=randomised controlled trial; TSANZ=Thoracic Society of Australia and New Zealand.
Although not widely-appreciated outside the discipline of respiratory medicine, low lung function (reflected in low spirometry values) is an independent risk factor for future morbidity and ‘all-cause’ mortality with an effect size (population attributable risk) larger than tobacco use and previous cardiovascular disease (24.7% vs 19.7% and 5.5% respectively).44 This finding is consistent across studies and settings (LMICs vs HICs, rural vs urban settings),44 even when FEV1 and forced vital capacity are in the clinically normal range (z-score>−2).44 Strategies that mitigate against developing low lung function would be transformative. All major parameters of lung function (measures reflecting obstruction and restriction defects) are mostly set in early childhood,18,45 but can improve significantly with optimal care,46 as can its trajectory into adulthood.18,47
While many factors influence lung function in childhood, several are non-modifiable (e.g. genetics) and others (e.g. social determinants) may take years before an effect is seen. ALRIs are one of the most important modifiable risk factors that can negatively impact upon future lung function.18,48,49 Furthermore, ALRIs are associated with future lung disease, including bronchiectasis.50,51
In addition, ALRIs are a main cause of hospitalisation (and mortality) globally in children aged<5-years,52,53 even in HICs e.g. the United States54 and Australia.55,56 Even with high vaccination rates and near universal breastfeeding among Indigenous infants in the Northern Territory of Australia, one in five are hospitalised in their first year of life.56 Thus, there is a universal need for novel evidence-based strategies to reduce ALRIs (and their recurrences) in infants and children to prevent subsequent low lung function and to decrease the overall global burden of bronchiectasis.1,2,5 These interventions will need to be suitable for administration in the very young, to act within a short timeframe, and their delivery feasible, especially for underserved populations.
Biologically, the negative impact of ALRIs on future lung health is plausible as human lung development continues for at least 7-years after birth with maximal postnatal lung growth occurring in the first 2-years of life.20 Early insults during this period, when the postnatal lung is most susceptible to injury, may impair the lung growth trajectory with long-term adverse effects.20 Consequently, intervention in early life is critical for improving overall lung health in children and adults.20,47
The impact of chronic wet cough, the key symptom of chronic suppurative lung diseases (PBB and bronchiectasis, Table 1),1,5,43,57 is compounded by low lung function and can also accelerate lung function decline.5 Our framework therefore includes studies that address preventive strategies that are feasible within a short timeframe (Table 1). These include randomised controlled trials (RCTs) on novel intervention that can prevent early ALRIs (maternal immunisation,58 pre- and post-natal vitamin D supplementation59 and novel probiotics), or its recurrence (azithromycin to prevent ALRI-hospitalisations60 or recurrent PBB/bronchiectasis61).
Theme B: Improving diagnosis and awareness and better disease characterisation of chronic wet cough and bronchiectasis (secondary prevention)Prompt diagnosis (from early detection) and optimal treatment are crucial in reducing the bronchiectasis disease burden.1,2 This requires better recognition and management of chronic wet cough, the critical and most common symptom of childhood bronchiectasis.1 Furthermore, the need to address this symptom is also important as bronchitis in adults (manifested as chronic wet cough) is associated with premature death62 and accelerated lung function trajectory decline.18
Most people with bronchiectasis have had a wet/productive cough for many years before it is diagnosed,22,43,63 and delays in diagnosis still occur.64,65 Cough duration correlates with poorer lung function and worse radiographic bronchiectasis scores.22,63 Better cough management (e.g. by using evidence-based cough guidelines) improves the early detection of underlying illness and its clinical outcomes,57,66,67 and is very important for prevention, earlier diagnosis and management of bronchiectasis43,68 and future lung health.1
Better disease characterisation of childhood bronchiectasis is priorities for both consumers and clinicians.28 This necessitates prospective studies identifying phenotypes and endotypes. Adult bronchiectasis data on phenotypes (e.g. frequent exacerbators) and endotypes (airway microbiota-inflammation) are emerging to provide a positive clinical impact,69,70 but no such prospective childhood bronchiectasis pheno-endotype data exist currently.
Completed studies in this theme: (i) consolidating the definition of paediatric radiographic bronchiectasis to improve its diagnosis,71 (ii) identifying and defining acute bronchiectasis exacerbations and its phenotypes,72,73 and (iii) healthcare costs related to childhood bronchiectasis32 (Table 1).
Further work includes identifying clinical phenotypes of bronchiectasis, as well as microbiota endotypes, that will predict the prognosis of PBB and bronchiectasis in children. There are limited prospective data on the composition and role of microbial communities in the lower airways of children with PBB74 or bronchiectasis.1 The European Respiratory Society (ERS) PBB statement has a research priorities list74 that includes studying the influence of respiratory microbiota upon the pathway of chronic cough to disease recurrence and progression (to bronchiectasis) so as to: (i) identify susceptible children and; (ii) develop novel treatment and/or prevention strategies.
Theme C: Better management of bronchiectasis to improve short and long-term prognosis (secondary prevention)It is acknowledged that high-quality routine clinical care (e.g. attention to infection, airway clearance techniques and nutrition) and treating underlying aetiological disorders and extra-pulmonary co-morbidities are important.1 CF is a good example. Before CF transmembrane regulator (CFTR) modulator and other targeted therapies became available, good clinical care alone substantially improved CF clinical outcomes (e.g. better lung function of children aged 6-years and median life expectancy [25-years in 1982 to 50-years in 2014]).75
Current treatment recommendations for childhood bronchiectasis are based largely upon extrapolating results from studies in CF and adults with bronchiectasis, and/or expert opinion.1,2 The need to increase the evidence base for improving bronchiectasis management, especially in paediatrics is clear.1,5 Consequently, we are undertaking studies that evaluate the benefits (or otherwise) of using a bronchiectasis action management plan76 and erdosteine (a novel mucolytic) (Table 1).
To further inform clinical practice on defining the optimal duration for prescribing azithromycin in bronchiectasis, we undertook a secondary analysis of our 24-month RCT.77 The original RCT showed that oral azithromycin reduced by 50% the frequency of respiratory exacerbations, but was accompanied by increased carriage of macrolide-resistant bacterial pathogens.77 In our secondary analysis, we found that azithromycin is best used for at least 17-weeks and up to 62-weeks, as these periods provided maximum benefit for reducing exacerbation frequency.78 We also found that its effects may be modified positively by nasopharyngeal carriage of bacterial pathogens, and higher weight-for-height z-scores, but negatively by preterm birth (Table 1).78
We discovered previously that the presence of chronic cough post-ALRI hospitalisation in Indigenous children predicted future bronchiectasis51 and cough was under-recognised by Indigenous people living remotely.79,80 Our earlier study57 and a systematic review81 found using a chronic cough guideline improved early detection of underlying chronic respiratory illness and clinical outcomes.43 Utilising chronic cough guidelines could potentially prevent bronchiectasis (by optimal treatment of pre-bronchiectasis states1,82; Fig. 1). Thus, our projects include implementing a chronic cough evidence-based guideline to improve chronic cough management83 and implementing a programme to improve medical follow-up and health outcomes for Indigenous children hospitalised with ALRIs84 (Table 1).
There is also an unmet need to improve accessibility to educational, bronchiectasis-specific mobile phone applications (APP) and relevant primary healthcare staff. We provide a resource list and website links that are currently available and easily accessible for consumers and health professionals (Table 2).
Repository of resources available for paediatric bronchiectasis.
| Targeted for patients/parents |
| • Adolescent's experience with bronchiectasis https://europeanlung.org/en/people-and-partners/your-experiences/ed-powell-my-experience-of-bronchiectasis/ |
| • Airway clearance techniques for children https://bronchiectasis.com.au/paediatrics |
| • Education Mobile APP https://www.menzies.edu.au/page/Resources/Lung_Health_for_kids/ |
| • ERS guidelines lay summary https://europeanlung.org/wp-content/uploads/2021/08/Bronchiectasis-lay-guidelines.pdf |
| • Frequently asked questions on bronchiectasis https://www.improvebe.org/learning-from-families |
| • Indigenous specific resources |
| www.crelungs.org.au |
| www.telethonkids.org.au/contentassets/b50404b2050d4c1e92cb7225fc3c2047/guide-to-wet-cough-resources.pdf |
| • Patient journey vimeo.com/851484556/4c5deeef8b?share=copy |
| Targeted for healthcare professionals |
| • Guidelines on management of children with bronchiectasis: |
| ∘ International ERS guideline https://pubmed.ncbi.nlm.nih.gov/33542057/ |
| ∘ Educational ERS guideline https://pubmed.ncbi.nlm.nih.gov/35035559/ |
| ∘ Australia and New Zealand guideline https://pubmed.ncbi.nlm.nih.gov/36863703/ |
| ∘ Quality standards for managing bronchiectasis https://pubmed.ncbi.nlm.nih.gov/36865655/ |
| • Patient facing resources for clinicians to use: |
| ∘ Bronchiectasis Action Management Plan https://www.improvebe.org/_files/ugd/acf027_688b0468d24f46dbaca7b6ee639a5051.pdf |
| ∘ Recognising chronic wet cough in Indigenous children https://www.telethonkids.org.au/our-research/research-topics/wet-cough/ |
| • Parents and patients’ needs and priorities international road map https://pubmed.ncbi.nlm.nih.gov/34291113/ |
| • Training modules (online) for: |
| ∘ (i) Asthma Diagnosis and Interpretation of Spirometry and (ii) Improving Outcomes Through Culturally Secure Care (management of chronic wet cough, PBB and bronchiectasis) https://lungfoundation.com.au/health-professionals/training-and-events/training/chronic-cough/ |
| ∘ Effective engagement with Aboriginal parents’ podcasts: https://open.spotify.com/show/0E44yzXbjXmOgFIkBOSxtx |
| • Other generic resources |
| ∘ Links to other societies are listed at the bottom of the page of this website https://www.improvebe.org/learning-from-families |
| ∘ https://bronchiectasis.com.au/ |
These resources were developed and evolved over almost three decades to provide clinical services and to facilitate translational research with children.68,93
The management and clinical outcomes of children with bronchiectasis have improved substantially. For example, the historical ‘destroyed lung’ described previously in Indigenous children85 is now rare and their lung function has recovered markedly.46,86 In the early 2000s, it was common to see lung function in children within the 30–60% predicted range at the time of receiving their bronchiectasis diagnosis.87 Currently, most Indigenous children with bronchiectasis that is managed optimally have lung function within the clinically normal range.46,86
Despite this progress, much more still needs to be done. We need resources to implement a plan to leverage what has been achieved by research to date. Globally, we also need to engage communities and their leaders to enable access and delivery of culturally appropriate care. Addressing social determinants of health in underserviced, disadvantaged populations remain an issue that needs specific on-going attention. Furthermore, there are many other potential contributors to disease (e.g. genetics, epigenetics, nutritional support, etc.) that are beyond the scope of our CRE and remit of our paper.
Disease-specific QoL tools are more sensitive than generic ones and are being considered increasingly as essential when evaluating health and intervention trials. QoL tools designed for adults are inappropriate for children.88 Since QoL measures are also considered the most important outcome for patients and parents of children with bronchiectasis,89 development of a paediatric bronchiectasis-specific QoL is now crucial. In addition, the priorities of parental needs and research priorities documented in an international survey28 should be used to guide research and clinical efforts to improve the lives of children with bronchiectasis and their families (Table 3).
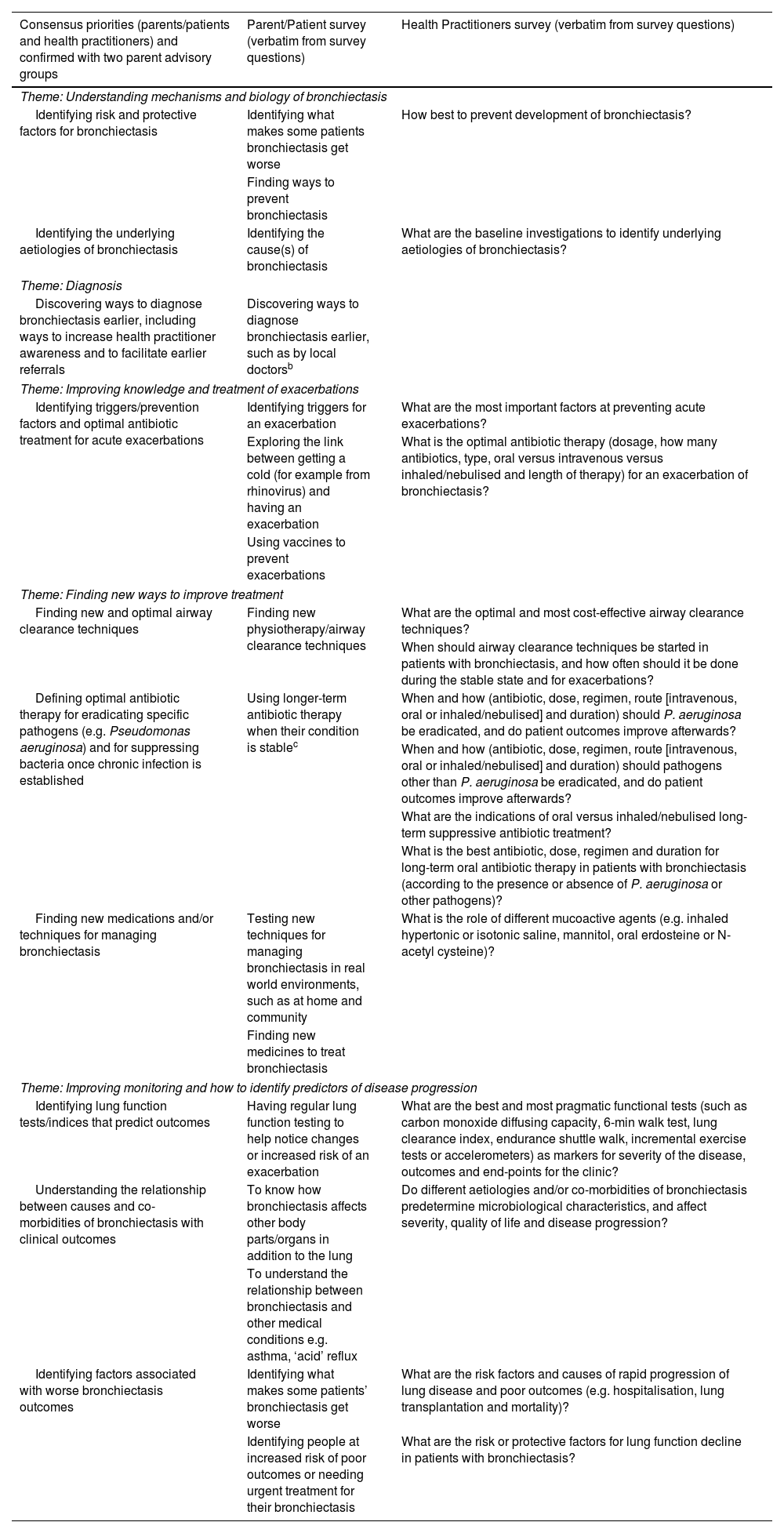
The top 10 research priorities derived from an international survey and consensus.a
| Consensus priorities (parents/patients and health practitioners) and confirmed with two parent advisory groups | Parent/Patient survey (verbatim from survey questions) | Health Practitioners survey (verbatim from survey questions) |
|---|---|---|
| Theme: Understanding mechanisms and biology of bronchiectasis | ||
| Identifying risk and protective factors for bronchiectasis | Identifying what makes some patients bronchiectasis get worse | How best to prevent development of bronchiectasis? |
| Finding ways to prevent bronchiectasis | ||
| Identifying the underlying aetiologies of bronchiectasis | Identifying the cause(s) of bronchiectasis | What are the baseline investigations to identify underlying aetiologies of bronchiectasis? |
| Theme: Diagnosis | ||
| Discovering ways to diagnose bronchiectasis earlier, including ways to increase health practitioner awareness and to facilitate earlier referrals | Discovering ways to diagnose bronchiectasis earlier, such as by local doctorsb | |
| Theme: Improving knowledge and treatment of exacerbations | ||
| Identifying triggers/prevention factors and optimal antibiotic treatment for acute exacerbations | Identifying triggers for an exacerbation | What are the most important factors at preventing acute exacerbations? |
| Exploring the link between getting a cold (for example from rhinovirus) and having an exacerbation | What is the optimal antibiotic therapy (dosage, how many antibiotics, type, oral versus intravenous versus inhaled/nebulised and length of therapy) for an exacerbation of bronchiectasis? | |
| Using vaccines to prevent exacerbations | ||
| Theme: Finding new ways to improve treatment | ||
| Finding new and optimal airway clearance techniques | Finding new physiotherapy/airway clearance techniques | What are the optimal and most cost-effective airway clearance techniques? |
| When should airway clearance techniques be started in patients with bronchiectasis, and how often should it be done during the stable state and for exacerbations? | ||
| Defining optimal antibiotic therapy for eradicating specific pathogens (e.g. Pseudomonas aeruginosa) and for suppressing bacteria once chronic infection is established | Using longer-term antibiotic therapy when their condition is stablec | When and how (antibiotic, dose, regimen, route [intravenous, oral or inhaled/nebulised] and duration) should P. aeruginosa be eradicated, and do patient outcomes improve afterwards? |
| When and how (antibiotic, dose, regimen, route [intravenous, oral or inhaled/nebulised] and duration) should pathogens other than P. aeruginosa be eradicated, and do patient outcomes improve afterwards? | ||
| What are the indications of oral versus inhaled/nebulised long-term suppressive antibiotic treatment? | ||
| What is the best antibiotic, dose, regimen and duration for long-term oral antibiotic therapy in patients with bronchiectasis (according to the presence or absence of P. aeruginosa or other pathogens)? | ||
| Finding new medications and/or techniques for managing bronchiectasis | Testing new techniques for managing bronchiectasis in real world environments, such as at home and community | What is the role of different mucoactive agents (e.g. inhaled hypertonic or isotonic saline, mannitol, oral erdosteine or N-acetyl cysteine)? |
| Finding new medicines to treat bronchiectasis | ||
| Theme: Improving monitoring and how to identify predictors of disease progression | ||
| Identifying lung function tests/indices that predict outcomes | Having regular lung function testing to help notice changes or increased risk of an exacerbation | What are the best and most pragmatic functional tests (such as carbon monoxide diffusing capacity, 6-min walk test, lung clearance index, endurance shuttle walk, incremental exercise tests or accelerometers) as markers for severity of the disease, outcomes and end-points for the clinic? |
| Understanding the relationship between causes and co-morbidities of bronchiectasis with clinical outcomes | To know how bronchiectasis affects other body parts/organs in addition to the lung | Do different aetiologies and/or co-morbidities of bronchiectasis predetermine microbiological characteristics, and affect severity, quality of life and disease progression? |
| To understand the relationship between bronchiectasis and other medical conditions e.g. asthma, ‘acid’ reflux | ||
| Identifying factors associated with worse bronchiectasis outcomes | Identifying what makes some patients’ bronchiectasis get worse | What are the risk factors and causes of rapid progression of lung disease and poor outcomes (e.g. hospitalisation, lung transplantation and mortality)? |
| Identifying people at increased risk of poor outcomes or needing urgent treatment for their bronchiectasis | What are the risk or protective factors for lung function decline in patients with bronchiectasis? | |
Additional resources are needed for community consultation, patient care, healthcare systems and research to achieve equity with other chronic lung disorders (e.g. asthma, COPD, CF). Also, despite the known global burden of lung diseases, it receives proportionately less research funding and public attention than other important chronic diseases (e.g. cancer, cardiovascular disease, diabetes).90
ConclusionsBronchiectasis is the third most common chronic airway disease, behind asthma and COPD.3 It affects all age groups and socioeconomic strata with the greatest burden in disadvantaged communities. Although bronchiectasis is most often diagnosed in older adults, more than half will have symptoms dating back to childhood.22,23 Furthermore, patients with symptoms from childhood have more advanced disease than those whose bronchiectasis only became apparent during their adult years.22,23 This is important, as bronchiectasis is potentially reversible in children when diagnosed early and treated optimally by multi-disciplinary healthcare teams. Timely treatment may also reduce the future bronchiectasis burden in adults.
Achieving these goals requires additional investment in (i) educating communities and healthcare professionals about the clinical significance of chronic wet/productive cough in children, (ii) funding research to identify modifiable proximal risk factors for bronchiectasis and design interventions that either prevent bronchiectasis developing or effectively managing it should it become established, and (iii) providing accessible and culturally-appropriate healthcare services to identify those at risk of bronchiectasis, and promptly diagnosing and treating those who have this chronic pulmonary disorder. We have focused on feasible strategies achievable within a short timeframe but there is also an on-going need to address complex issues (including social determinants) and other potential contributing factors (e.g. genetic and epigenetic associations). Finally, in this review, we have not focused on current management and instead refer readers to recent articles and existing guidelines (including their supplementary material) on the management of bronchiectasis in children/adolescents.1,2,24
Authors’ contributionsThe collective works reported are combined efforts of all the authors and their teams. AC conceptualised the framework and manuscript, and wrote the first draft which KG edited and then circulated to all (writing and extended groups) who amended the manuscript.
FundingThe workshop was supported by a National Health and Medical Research Council (NHMRC) Centre of Research Excellence (CRE) grant for paediatric bronchiectasis, especially in Aboriginal and Torres Strait Islanders grant (GNT1170958). ABC (L3, GNT2025379) and SCD are supported by NHMRC Investigator fellowships. JMM, STY and DFW are supported by NHMRC CRE (GNT1170958) fellowships. AS is supported by a Medical Research Future Fund (MRFF) Fellowship (APP1193796).
Conflict of interestABC, STY, SCD, KG, AS, PSM, MT, GBM and JMM report grants from the NHMRC and NHMRC managed grants (MRFF), Australia, during the conduct of the CRE. ABC is also an independent data management committee member for clinical trials for Moderna (COVID-19 vaccine) and of an unlicensed vaccine (GlaxoSmithKline), and monoclonal antibody (AstraZeneca), received fees to the institution for consulting on study designs for Zambon and Boehringer Ingelheim, and personal fees for being an author of two UpToDate chapters. SCD holds an investigator-initiated grant from GlaxoSmithKline and AstraZeneca for unrelated research. AS has received honoraria as advisory board member for Vertex Pharmaceuticals. All other authors have nothing to declare.
We are very grateful to the First Nations Reference Group and the Parent Advisory Group who over many years, have provided sound advice, and worked consistently with us to ensure our research and its translation reflect the wishes of the community and Indigenous people.
CRE extended group are other investigators in the grant, higher degree research scholars, staff, collaborators and parents who attended the workshop held at the Children's Centre for Health Research, Brisbane on 24–26th August 2023. They contributed to the grant and/or the discussions that helped formulate this paper: Atack JM, Azam S, Baxter C, Binks MJ, Bleakley AS, Boonjindasup W, Bui DS, Buntain HM, Cheng AC, Cheng MF, D’Antoine H, Da Silva M, Emmett L, Fuery A, Gadoury A, Garriga-Grimau L, Goyal V, Gray E, Hill J, Hoffman LR, Howard DR, Ilhan H, Johnstone E, Kapur N, Kok HC, Laird PJ, Lau G, Lee B, Marsh RL, Mandal PK, Marchant H, Mason DM, McElrea MM, McPhail SM, Mitchell P, Mitchell R, O’Farrell HE, Outridge R, Petsky HL, Perret JL, Pratt A, Phipps S, Ramsey K, Roberts JM, Robinson PD, Schutz K, Smith-Vaughan HS, Torzillo PJ, Thomas R, Upham JW, Vicendese D, Versteegh LA, West NP, Wilson C.